Botanic characteristics—
A mixture of segments of the roots and rhizomes. The root segments are mostly curved and flexuous, occasionally branched, up to 15 cm in length and usually from 3 to 6.5 mm in diameter, but may be up to 9 mm in diameter, grayish, grayish brown, or reddish brown, the reddish brown type often having light-colored abrasions, transverse ridges about 0.5 to 1.0 mm wide that extend about halfway around the circumference of the root and fade at their tapering extremities into the general surface, with from 1 to 6 of these ridges per cm, and annulations sometimes seen at irregular intervals. The rhizomes are cylindrical, about 2 mm thick, finely longitudinally wrinkled, with a few elliptical scars. The odor is distinctive; the dust is sternutatory.
Histology—
At the center of the root is a well-defined primary xylem but no pith. Surrounding this is a dense wood of secondary xylem crossed by medullary rays. These elements are all lignified. External to the wood is a narrow band of secondary phloem and a wide parenchymatous phelloderm surrounded by a narrow layer of cork a few cells thick. The secondary xylem consists of narrow, bordered-pitted tracheidal vessels and tracheids in combination with xylem parenchyma. The latter have simple pits and contain starch grains. Starch is present also in the medullary rays. The phloem occurs as small groups of sieve tissue embedded in parenchyma. The wide phelloderm consists of round-celled cellulose parenchyma filled with starch grains and a few idioblasts, each of which contains a bundle of acicular raphides of calcium oxalate crystals about 30 to 80 µm long. The starch grains are rarely single but usually occur as 2 to 4 and sometimes 8 in a clump. Individual grains measure up to 22 µm in diameter.
The rhizome differs from the root in having a ring of xylem around a large pith. The pericycle contains characteristic sclerenchymatous cells. Spiral vessels are found in the protoxylem. The pith is composed of pitted parenchyma, which is somewhat lignified.
Assay for total ether-soluble alkaloids—
[NOTE—It is important that the ether used in this assay shall have been shown by test to be free from peroxides within 24 hours prior to use.
] The alkaloids may be extracted by either of the methods given in the following two paragraphs.
I—
To 10 g of finely powdered Ipecac, in a suitable container, add 100 mL of ether, accurately measured at 25

, insert the stopper in the container tightly, shake the mixture thoroughly, and allow it to stand for 5 minutes. Then add 10 mL of 6 N ammonium hydroxide, close again tightly, shake it for 1 hour in a mechanical shaker or intermittently during 2 hours, and allow to stand overnight at a temperature not exceeding 25

. Again shake the mixture intermittently during 30 minutes, and allow the drug to settle at 25

. Transfer to a separator a 50.0-mL aliquot of the clear, supernatant, representing 5 g of Ipecac.
II—
Place 5 g of the finely powdered Ipecac in a continuous-extraction thimble. Add enough ether to cover the powder, and allow to stand for 10 minutes with occasional stirring. Add 3 mL of ammonium hydroxide, mix, and allow to stand overnight. Cover the drug with a pledget of cotton, pack well, and extract with ether for 5 hours. Transfer the ether extract to a separator.
Extract the alkaloids from the ether with 2 N sulfuric acid, using at first 15 mL, or more, if necessary, to ensure an acid reaction, then successive 10-mL portions until extraction is complete, and filtering all extracts through the same filter into a second separator. To the combined acid solutions add about an equal volume of ether, render the mixture distinctly alkaline with 6 N ammonium hydroxide (at least pH 10, by test paper), and extract with successive portions of ether until the last extract shows not more than a slight turbidity when treated as follows: Evaporate 1 mL of the last extraction, dissolve the residue in 0.5 mL of 0.5 N hydrochloric acid, and add 1 drop of mercuric iodide TS.
Filter each portion of the ether extract into a flask or beaker, and carefully evaporate the combined ether extracts on a steam bath almost to dryness. Add 5 mL of ether and 10.0 mL of 0.1 N sulfuric acid VS, and heat on a steam bath to dissolve the alkaloids and to remove all the ether. Cool, add 15 mL of water, then add
methyl red TS, and titrate the excess acid with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sulfuric acid is equivalent to 24.0 mg of the total ether-soluble alkaloids of ipecac, calculated as emetine (C
29H
40N
2O
4).
Assay for emetine and cephaeline—
Standard preparation—
Accurately weigh a suitable quantity of
USP Emetine Hydrochloride RS, and dissolve in 0.5 N sulfuric acid. Dilute quantitatively and stepwise with the same dilute sulfuric acid to obtain a solution having a known concentration equivalent to about 50 µg of emetine per mL.
Assay preparation—
Prepare a
Test Sample as directed in
Methods of Analysis under
Articles of Botanical Origin  561
561
. Transfer to a 150-mL beaker about 200 mg, accurately weighed, of the fine powder. Add 2 mL of dimethyl sulfoxide, mix with a flattened stirring rod to assure complete wetting of the powder, and allow to stand for about 30 minutes. Add 2 mL of water and about 1 g of sodium bicarbonate, and mix.
Phosphate buffer—
Prepare approximately 0.5 M solutions of monobasic potassium phosphate (containing 5.1 g per 75 mL) and dibasic potassium phosphate (containing 2.2 g per 25 mL). Mix 3 volumes of 0.5 M monobasic potassium phosphate with 1 volume of 0.5 M dibasic potassium phosphate, and adjust by the addition of one or the other of the solutions to a pH of 6.0 ± 0.05. Dissolve 7.5 g of potassium chloride in 100 mL of the resulting solution.
Citric acid buffer—
Prepare approximately 0.5 M solutions of sodium citrate (containing 6.5 g per 50 mL) and citric acid (containing 4.8 g per 50 mL). Mix equal volumes of these solutions, and adjust by addition of one or the other of the solutions to a pH of 4.0 ± 0.05.
Chromatographic columns—
For each column, pack a pledget of fine glass wool in the base of a chromatographic tube (25- × 200-mm test tube to which is fused a 5-cm length of 7-mm tubing) with the aid of a tamping rod having a disk with a diameter about 1 mm less than that of the tube.
Prepare Column I as follows. To the Assay preparation add 6 g of purified siliceous earth, mix, transfer the mixture to the column, scrub the beaker with about 1 g of the purified siliceous earth, transfer this to the top of the column, and tamp. Prepare Column II using 3 g of the purified siliceous earth and 2 mL of Phosphate buffer; prepare Column III using 2 mL of Citric acid buffer and 3 g of the purified siliceous earth; and prepare Column IV using 2 mL of sodium hydroxide solution (1 in 50) and 3 g of the purified siliceous earth. Pack a pledget of glass wool on the top of each column.
Procedure—
[NOTE—Use water-saturated solvents throughout this procedure. Rinse the tips of the chromatographic columns before discarding them.] Mount Columns I and II so that the effluent from Column I flows onto Column II. Pass three 50-mL portions of ether through the columns, and discard Column I and the eluate. Mount Column III below Column II and pass three 50-mL portions of a mixture of 1 volume of ether and 3 volumes of chloroform through the columns. Discard Column II and the eluate. Wash Column III with 25 mL of the ether–chloroform mixture, followed by 25 mL of a mixture of equal volumes of ether and isooctane, and discard the washings. Wash Column IV with 20 mL of a 1 in 50 solution of triethylamine in the ether–isooctane mixture, and discard the washing. Mount Column IV below Column III, and place as a receiver under Column IV a 125-mL separator containing 15 mL of 4 N sulfuric acid. Pass through the columns 10 mL of a 1 in 5 solution of triethylamine in the ether–isooctane mixture, followed by three 10-mL portions of a 1 in 50 solution of triethylamine in the ether–isooctane mixture. Discard Column III, and pass through Column IV 20 mL of the 1 in 50 solution of triethylamine in the ether–isooctane mixture. Shake the separator, allow the phases to separate, and transfer the aqueous extract to a 50-mL volumetric flask. Extract with two additional 10-mL portions of 0.5 N sulfuric acid, combining the extracts in the volumetric flask. Add 0.5 N sulfuric acid to volume, and mix (emetine solution).
Elute Column IV with 75 mL of chloroform, collecting the eluate in a 250-mL separator containing 150 mL of ether. Discard Column IV. Extract with one 20-mL and then with two 10-mL portions of 0.5 N sulfuric acid, collecting the extracts in a 50-mL volumetric flask. Rinse the stem of the separator, add the acid to volume, and mix (cephaeline solution).
Concomitantly determine the absorbances of the emetine solution, the cephaeline solution, and the Standard preparation in 1-cm cells at the wavelength of maximum absorbance at about 283 nm and at 350 nm, with a suitable spectrophotometer, using 0.5 N sulfuric acid as the blank.
Calculate the quantity, in mg, of emetine in the portion of Ipecac taken by the formula:
0.05
C(
A283 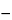 A350
A350)
U / (
A283 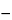 A350
A350)
S,
in which C is the concentration, in µg per mL, of emetine in the Standard preparation; and the parenthetic expressions are the differences in the absorbances of the solution of emetine from the Assay preparation (U) and the Standard preparation (S), respectively, at the wavelengths indicated by the subscripts.
Calculate the quantity, in mg, of cephaeline in the portion of Ipecac taken by the formula:
0.971(0.05
C)(
A283 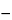 A350
A350)
U / (
A283 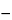 A350
A350)
S,
in which 0.971 is the ratio of the molecular weight of cephaeline to that of emetine; C is as defined above; and the parenthetic expressions are the differences in the absorbances of the solution of cephaeline from the Assay preparation (U) and the Standard preparation (S), respectively, at the wavelengths indicated by the subscripts.

