Packaging and storage—
Preserve in single-dose glass or plastic containers, of not larger than 1-L size. Glass containers are preferably of Type I or Type II glass.
Labeling—
Label it to indicate that no antimicrobial or other substance has been added, and that it is not suitable for intravascular injection without first having been made approximately isotonic by the addition of a suitable solute.
pH 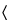 791
791 :
:
between 5.0 and 7.0 in a solution containing 0.3 mL of saturated potassium chloride solution per 100 mL of test specimen.
Ammonia—
For containers having a fill volume of less than 50 mL, dilute 50 mL of it with 50 mL of
High-Purity Water (see
Reagents under
Containers 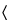 661
661
), and use this dilution as the test solution; where the fill volume is 50 mL or more, use 100 mL of it as the test solution. To 100 mL of the test solution add 2 mL of alkaline mercuric-potassium iodide TS: any yellow color produced immediately is not darker than that of a control containing 30 µg of added ammonia (furnished by using 1.76 mL of 1.0 N ammonium hydroxide) in 100 mL of
High-Purity Water. This corresponds to a limit of 0.6 mg per L for containers having a fill volume of less than 50 mL and 0.3 mg per L where the fill volume is 50 mL or more.
Chloride—
To 20 mL in a color-comparison tube add 5 drops of nitric acid and 1 mL of
silver nitrate TS, and gently mix: any turbidity formed within 10 minutes is not greater than that produced in a similarly treated control consisting of 20 mL of
High-Purity Water (see
Reagents under
Containers  661
661
) containing 10 µg of chloride (0.5 mg per L), viewed downward over a dark surface with light entering the tubes from the sides.
Oxidizable substances—
To 100 mL add 10 mL of 2 N sulfuric acid, and heat to boiling. For Sterile Water for Injection in containers having a fill volume of less than 50 mL, add 0.4 mL of 0.1 N potassium permanganate, and boil for 5 minutes; where the fill volume is 50 mL or more, add 0.2 mL of 0.1 N potassium permanganate, and boil for 5 minutes. If a precipitate forms, cool in an ice bath to room temperature, and pass through a sintered-glass filter: the pink color does not completely disappear.

