Bacterial Alkaline Protease Preparation—
Use a suitable grade.
Barbital Sodium,
 C8
C8H
11N
2NaO
3—
206.2


[
144-02-5]—White, crystalline powder or colorless crystals. Freely soluble in water; slightly soluble in alcohol. Use a suitable reagent grade.
Barium Acetate,
 C4
C4H
6BaO
4—
255.43


[
543-80-6]—Use ACS reagent grade.
Barium Chloride,

BaCl
2·2H
2O—
244.26—Use ACS reagent grade.
Barium Chloride, Anhydrous,

BaCl
2—
208.23—This may be made by drying barium chloride in thin layers at 125

until the loss in weight between two successive, 3-hour drying periods does not exceed 1%.
Barium Chloride Dihydrate
—Use Barium Chloride.
Barium Hydroxide,

Ba(OH)
2·8H
2O—
315.46—Use ACS reagent grade.
Barium Nitrate,

Ba(NO
3)
2—
261.34—Use ACS reagent grade.
Beef Extract
—A concentrate from beef broth obtained by extraction from fresh, sound, lean beef by means of cooking with water and evaporating the broth at a low temperature, usually in vacuum, until a thick, pasty residue results. Yellowish brown to dark brown, slightly acid, pasty mass having an agreeable meat-like odor. Store it in tight, light-resistant containers.
For the following tests, prepare a test solution by dissolving 25 g in water to make 250 mL of a practically clear and practically sediment-free solution.
Assay for nitrogen content of alcohol-soluble substances—
Place a portion of the alcohol filtrate and washings remaining from the test for
Alcohol-insoluble substances, corresponding to 1 g of the alcohol-soluble solids, in a 500-mL Kjeldahl flask. Add about 10 g of powdered potassium sulfate and 20 mL of sulfuric acid. Heat the mixture at a low temperature until frothing ceases, then raise the temperature and boil until the mixture acquires a pale yellow color or becomes practically colorless. Cool the flask, add about 250 mL of water, and cautiously add sodium hydroxide solution (3 in 10) until the contents are alkaline, then add 5 mL more. Connect the flask at once by means of a spray trap to a condenser, the lower outlet tube of which dips beneath the surface of 50.0 mL of 0.1 N sulfuric acid VS contained in a receiving flask. Distill the mixture until about 100 mL of distillate has been collected in the acid. Add
methyl red TS, and titrate the excess acid with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sulfuric acid is equivalent to 1.401 mg of N. Not less than 60 mg of nitrogen is found.
Assay for nitrogen as ammonia—
To 100 mL of
test solution, contained in a 500-mL Kjeldahl flask, add 5 g of barium carbonate and 100 mL of water, and by means of a spray trap, connect the flask to a condenser, the lower outlet tube of which dips beneath the surface of 50.0 mL of 0.1 N sulfuric acid VS contained in a receiving flask. Distill the mixture until about 100 mL of distillate has been collected, add
methyl red TS, and titrate the excess acid with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sulfuric acid is equivalent to 1.703 mg of NH
3. The amount of ammonia found does not exceed 0.35% of the total solids in the portion of
test solution taken.
Total solids—
Distribute 10 mL of
test solution over clean, dry sand or asbestos, tared in a porcelain dish, and dry at 105

for 16 hours: the residue weighs not less than 750 mg (75%).
Residue on ignition—
Incinerate the residue obtained in the test for Total solids by heating the dish to a dull-red heat: the residue does not exceed 30% of the total solids.
Chlorides calculated as sodium chloride—
Dissolve the ash obtained in the test for
Residue on ignition in about 50 mL of water, and carefully transfer to a 100-mL volumetric flask. Add to the solution a few drops of nitric acid and 10.0 mL of 0.1 N silver nitrate VS. Add water to volume, and mix. Filter into a dry flask through a dry filter, rejecting the first 10 mL of the filtrate. To 50.0 mL of the subsequent filtrate add 1 mL of
ferric ammonium sulfate TS, and titrate with 0.1 N ammonium thiocyanate VS. Each mL of 0.1 N silver nitrate is equivalent to 5.844 mg of NaCl. The weight of chlorides calculated as sodium chloride obtained, when multiplied by 2, does not exceed 6% of the total solids.
Alcohol-insoluble substances—
Transfer 25 mL of
test solution to a 100-mL conical flask, add 50 mL of alcohol, and shake thoroughly. Collect the precipitate on a counterpoised filter, wash it three times with a mixture of 2 volumes of alcohol and 1 volume of water, and dry at 105

for 2 hours: the weight of the precipitate, representing the alcohol-insoluble solids, does not exceed 10% of the total solids in the portion of
test solution taken.
Nitrate—
Boil 10 mL of test solution for 1 minute with 1.5 g of activated charcoal, add water to replace that lost by evaporation, filter, and add 1 drop of the filtrate to 3 drops of a 1 in 100 solution of diphenylamine in sulfuric acid: no blue color is produced.
Change to read:
Benzaldehyde,

C
7H
6O—
106.12—Colorless, strongly refractive liquid.

 USP29
USP29 Soluble in water; miscible with alcohol, with ether, and with fixed and volatile oils.
Assay—
Pipet about 1 mL into a tared, glass-stoppered weighing bottle, and weigh accurately. Loosen the stopper, and transfer both the weighing bottle and its contents to a 250-mL conical flask containing 25 mL of a hydro-alcoholic solution of hydroxylamine hydrochloride (prepared by dissolving 34.7 g of hydroxylamine hydrochloride in 160 mL of water, then adding alcohol to make 1000 mL, and neutralizing to bromophenol blue by the addition of
sodium hydroxide TS). Using a graduated cylinder to measure the volume, rinse the sides of the flask with an additional 50 mL of this reagent solution. Allow the solution to stand for 10 minutes, add 1 mL of
bromophenol blue TS, and titrate the liberated hydrochloric acid with 1 N sodium hydroxide VS. Perform a blank determination with the same quantities of the same reagents, and make any necessary correction. Each mL of 1 N sodium hydroxide consumed is equivalent to 106.1 mg of C
7H
6O. Not less than 98% is found.
Hydrocyanic acid—
Shake 0.5 mL with 5 mL of water, add 0.5 mL of
sodium hydroxide TS and 0.1 mL of
ferrous sulfate TS, and warm the mixture gently. Add a slight excess of hydrochloric acid: no greenish-blue color or blue precipitate is observed within 15 minutes.
Benzalkonium Chloride
—Use Benzalkonium Chloride (NF monograph).
Benzamidine Hydrochloride Hydrate,

C
7H
8N
2·HCl—
156.6


[
1670-14-0]—White to off-white powder. Use a suitable grade.
Benzanilide,

C
13H
11NO—
197.23—Off-white, light gray to grayish green powder. Insoluble in water; sparingly soluble in alcohol; slightly soluble in ether.
Solubility in acetone—
A 1.0-g portion dissolves completely in 50 mL of acetone to yield a clear solution.
Benzene,

C
6H
6—
78.11—Use ACS reagent grade.
Benzenesulfonamide,
 C6
C6H
5SO
2NH
2—
157.19—White to pale beige crystals.
Benzenesulfonyl Chloride,

C
6H
5SO
2Cl—
176.62—Colorless, oily liquid. Insoluble in cold water; soluble in alcohol and in ether. Solidifies at 0

.
Boiling range (Reagent test):

between 251

and 252

.
Benzhydrol
(
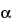 -Phenylbenzenemethanol
-Phenylbenzenemethanol),

C
13H
12O—
184.23—White to pale yellow crystals. Very slightly soluble in water; soluble in alcohol, in ether, and in chloroform.
Melting range 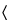 741
741 :
:

between 65

and 67

, but the range between beginning and end of melting does not exceed 2

.
Benzoic Acid,

C
6H
5COOH—
122.12—Use ACS reagent grade.
[NOTE—Benzoic Acid of a quality suitable as a primary standard is available from the National Institute of Standards and Technology, Office of Standard Reference Materials,
www.nist.gov, as standard sample No. 350.
]
Benzophenone,

(C
6H
5)
2CO—
182.22—White, crystalline powder.
p-Benzoquinone,

C
6H
4O
2—
108.09—Dark yellow powder having a green cast. Slightly soluble in water; soluble in alcohol, in ether, and in fixed alkali solutions. May darken on standing. Darkened material may be purified by sublimation in vacuum.
3-Benzoylbenzoic Acid,

C
14H
10O
3—
226.23—White to off-white powder.
Assay—
Prepare a mixture of 1% trifluoroacetic acid in water and 1% trifluoroacetic acid in acetonitrile (55:45) for the mobile phase. Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography 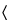 621
621
) equipped with a 230-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. The area of the C
14H
10O
3 peak is not less than 98.5% of the total peak area.
Benzoyl Chloride,

C
6H
5COCl—
140.57—Use ACS reagent grade.
N-Benzoyl-L-arginine Ethyl Ester Hydrochloride,

C
15H
22N
4O
3·HCl—
342.82—Determine suitability for use as a substrate as directed under
Crystallized Trypsin (USP monograph).
Benzoylformic Acid (phenylglyoxylic acid)

C
6H
5COCO
2H—
150.14—Powder. Soluble in methanol.
Change to read:
Benzphetamine Hydrochloride,

C
17H
21N·HCl—
275.82—White to off-white,

 USP29
USP29 crystalline powder. Freely soluble in water, in alcohol, and in chloroform; slightly soluble in ether.
Assay—
Dissolve about 500 mg, accurately weighed, in a mixture of 50 mL of glacial acetic acid and 10 mL of
mercuric acetate TS, add 1 drop of
crystal violet TS, and titrate with 0.1 N perchloric acid VS to a blue-green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 27.58 mg of C
17H
21N·HCl. Between 98.0% and 101.0%, calculated on the dried basis, is found.
Specific rotation  781
781 :
:
between +22

and +26

, determined in a solution containing 200 mg in 10 mL, the specimen having been previously dried in vacuum at 60

for 3 hours.
Loss on drying  731
731 —
—
Dry in vacuum at 60

for 3 hours: it loses not more than 1% of its weight.
2-Benzylaminopyridine,

C
12H
12N
2—
184.24—Use a suitable grade.
1-Benzylimidazole,

C
10H
10N
2—
158.20—White crystals.
Assay—
Transfer about 40 mg, accurately weighed, to a 100-mL beaker. Dissolve in 50 mL of glacial acetic acid. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically using a combination calomel-platinum electrode. Perform a blank determination and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 15.82 mg of C10H10N2. Not less than 99% is found.
Change to read:
Benzyltrimethylammonium Chloride,

C
6H
5CH
2N(CH
3)
3Cl—
185.69—Available as a 60% aqueous solution. Is clear and is colorless or not more than slightly yellow.

 USP29
USP29
Assay—
Pipet 2 mL into a 50-mL volumetric flask, and add water to volume. Pipet 20 mL of the solution into a 125-mL conical flask, add about 30 mL of water, then add 0.25 mL of dichlorofluorescein TS, and titrate with 0.1 N silver nitrate VS. Each mL of 0.1 N silver nitrate is equivalent to 18.57 mg of C6H5CH2N(CH3)3Cl. Between 59.5% and 60.5% is found.
Beta-lactamase
—Beta-lactamase is an enzyme produced by a variety of bacteria, but is usually obtained from culture filtrates of a strain of
Bacillus cereus. It has the specific property of inactivating penicillins and cephalosporins by splitting the bond linking the nitrogen of the thiazolidine to the adjacent carbonyl carbon.
It occurs in the form of small, brown, easily pulverizable pieces or granules. Freely soluble in water, forming a slightly opalescent solution that is practically neutral to litmus paper. Is precipitated from its water solutions by acetone, by alcohol, and by dioxane, and is inactivated by contact with these solvents. Is rapidly inactivated by ethyl acetate and is irreversibly destroyed at a temperature of about 80

.
Beta-lactamase is assayed by a procedure depending upon a determination of the amount of penicillin G potassium or penicillin G sodium destroyed at a pH of 7.0 in a solution of such concentration that the inactivation proceeds as a zero-order reaction.
Bibenzyl
(
Dibenzyl),

C
14H
14—
182.26—Colorless crystals. Freely soluble in chloroform and in ether; sparingly soluble in alcohol; practically insoluble in water.
Bile Salts
—A concentrate of beef bile, the principal constituent of which is sodium desoxycholate, determined as cholic acid. Soluble in water and in alcohol; the solutions foam strongly when shaken.
Insoluble substances—
Dissolve 5 g in 100 mL of dilute alcohol (84 in 100), warming if necessary to aid solution. Filter within 15 minutes through a tared filter, and wash with small portions of the dilute alcohol until the last washing is colorless or practically so, then dry the residue at 105

for 1 hour, and weigh: the weight of the residue does not exceed 0.1%.
Assay—
 STANDARD CHOLIC ACID SOLUTION—
STANDARD CHOLIC ACID SOLUTION—
Dissolve 50.0 mg of cholic acid, accurately weighed, in dilute acetic acid (6 in 10) to make 100 mL, and mix. Store in a refrigerator.
 PROCEDURE—
PROCEDURE—
Dissolve 1.0 g, accurately weighed, in 50 mL of dilute acetic acid (6 in 10). Filter the solution, if necessary, into a 100-mL volumetric flask, wash the original container and the filter with small portions of dilute acetic acid (6 in 10), add the same acetic acid to volume, and mix. Dilute 10 mL of this solution, accurately measured, with dilute acetic acid (6 in 10) to make 100 mL, and mix.
Pipet 1 mL each of the
Standard Cholic Acid Solution and the solution of the Bile Salts into two matched test tubes. To each tube add 1 mL, accurately measured, of freshly prepared furfural solution (1 in 100), immediately place the tubes in an ice-bath for 5 minutes, then add to each tube 13 mL, accurately measured, of dilute sulfuric acid, made by cautiously mixing 50 mL of sulfuric acid with 65 mL of water. Mix the contents of the tubes, and place them in a water bath maintained at a temperature of 70

for 10 minutes. Immediately transfer the tubes to an ice-bath for 2 minutes, then determine the absorbance of each solution at the wavelength of maximum absorbance at about 670 nm, with a suitable spectrophotometer. Calculate the quantity, in mg, of cholic acid (C
24H
40O
5) in the weight of the Bile Salts taken by the formula:
500(AU / AS),
in which AU and AS are the absorbances of the solutions from the Bile Salts and the Standard Cholic Acid Solution, respectively. Not less than 45% of cholic acid is found.
Change to read:
Biphenyl,

C
12H
10—
154.21—Colorless to white crystals or crystalline powder.

 USP29
USP29 Insoluble in water; soluble in alcohol and in ether. Boils at about 254

.
2,2¢-Bipyridine
(
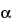
,
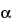 ¢
¢-
Dipyridyl),

C
10H
8N
2—
156.18—White or pink, crystalline powder. Soluble in water and in alcohol. Melts at about 69

, and boils at about 272

.
Sensitiveness—
Prepare the following solutions: (A)—Dissolve 350 mg of ferrous ammonium sulfate in 50 mL of water containing 1 mL of sulfuric acid, and add 500 mg of hydrazine sulfate, then add water to make 500 mL. For use, dilute this solution with water in the ratio of 1 in 100 mL. (B)—Dissolve 8.3 g of sodium acetate and 12 mL of glacial acetic acid in water to make 100 mL. Add 1 mL of a solution of the specimen (1 in 1000) to a mixture of 10 mL of water and 1 mL of each of solutions A and B: a pink color results immediately.
Solubility—
A 100-mg portion dissolves completely in 10 mL of water.
Residue on ignition (Reagent test):

not more than 0.2%.
4,4¢-Bis(4-amino-1-naphthylazo)-2,2¢-stilbenedisulfonic Acid,

C
34H
26N
6O
6S
2—
678.74—Use a suitable grade.
Bis(2-ethylhexyl) Maleate,

C
20H
36O
4—
340.50—Colorless to pale yellow, clear liquid. Miscible with acetone and with alcohol. Specific gravity about 0.945.
Assay—
Place about 2.5 g, accurately weighed, in a 250-mL flask, add 50.0 mL of 0.5 N alcoholic potassium hydroxide VS, and reflux for 45 minutes. Cool, add 0.5 mL of phenolphthalein TS, and titrate the excess alkali with 0.5 N hydrochloric acid VS. Perform a blank determination at the same time, using the same amount of 0.5 N alcoholic potassium hydroxide (See
Residual Titrations under
Titrimetry  541
541
). The difference, in mL, between the volumes of 0.5 N hydrochloric acid consumed in the test titration and blank titration, multiplied by 85.1, represents the quantity, in mg, of bis(2-ethylhexyl) maleate in the portion taken. Not less than 97% is found.
Bis(2-ethylhexyl) Phthalate,

C
6H
4-1,2-[COOCH
2(C
2H
5)CH(CH
2)
3CH
3]
2—
390.56—Colorless to light yellow liquid.
Bis(2-ethylhexyl) Sebacate
(
Dioctyl Sebacate),

C
8H
17OOC(CH
2)
8COOC
8H
17—
426.67—Pale straw-colored liquid. Insoluble in water. Refractive index about 1.448. Suitable for use in gas chromatography.
Boiling range:

between 243

and 248

at 5 mm of mercury.
Bis(2-ethylhexyl)(phosphoric Acid)
[
Bis-(
2-ethylhexyl)
Phosphate],

[CH
3(CH
2)
3CH(C
2H
5)CH
2]
2HPO
4—
322.42—Light yellow, viscous liquid. Insoluble in water; freely soluble in chloroform and in ethyl acetate. Refractive index: about 1.443. Specific gravity: about 0.997.
Assay—
Dissolve about 250 mg, accurately weighed, in 50 mL of dimethylformamide, add 3 drops of a 1 in 100 solution of thymol blue in dimethylformamide, and titrate with 0.1 N sodium methoxide VS to a blue endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium methoxide is equivalent to 32.24 mg of (C8H17)2HPO4. Between 95% and 105% is found.
Solubility—
One volume dissolves in 9 volumes of chloroform to yield a clear solution, and 1 volume dissolves in 9 volumes of ethyl acetate to yield a clear solution.
Color—
A 1 in 100 solution in chloroform exhibits an absorptivity of not more than 0.03 at 420 nm.
Bismuth Nitrate Pentahydrate,

Bi(NO
3)
3·5H
2O—
485.07


[
10035-06-0]—Use ACS reagent grade.
Bismuth Sulfite Agar
—Use a suitable grade.
Bis(trimethylsilyl)acetamide
(
N,
O-Bis(
trimethylsilyl)
-acetamide; BSA),

CH
3CON[Si(CH
3)
3]
2—
203.43—Clear, colorless liquid. Readily hydrolyzes when exposed to moist air. Handle under nitrogen, and store in a cool place.
Assay—
Not less than 90% of CH
3CON[Si(CH
3)
3]
2, a suitable gas chromatograph equipped with a thermal conductivity detector being used. The following conditions are suitable and provide a retention time of approximately 15 minutes.
 COLUMN:
COLUMN:

3-mm × 1.83-m stainless steel containing 5% phase G1 on support S1A.
 INJECTION TEMPERATURE:
INJECTION TEMPERATURE:

160

.
 COLUMN TEMPERATURE:
COLUMN TEMPERATURE:

90

, programmed to rise 4

per minute to 160

.
 CARRIER GAS:
CARRIER GAS:

Helium.
Bis(trimethylsilyl)trifluoroacetamide
(
BSTFA),

CF
3CON[Si(CH
3)
3]
2—
257.40—Clear, colorless liquid. Readily hydrolyzes when exposed to moist air. Store in a cool place.
Assay—
Not less than 98% of CF
3CON[Si(CH
3)
3]
2, a suitable gas chromatograph equipped with a thermal conductivity detector being used. The following conditions are suitable and provide a retention time of approximately 15 minutes.
 COLUMN:
COLUMN:

3-mm × 1.83-m stainless steel containing 5% phase G1 on support S1A.
 INJECTION TEMPERATURE:
INJECTION TEMPERATURE:

170

.
 COLUMN TEMPERATURE:
COLUMN TEMPERATURE:

70

, programmed to rise 4

per minute to 140

.
 CARRIER GAS:
CARRIER GAS:

Helium.
Bis(trimethylsilyl)trifluoroacetamide with Trimethylchlorosilane
—Use a suitable grade.
Blood
(for carbon monoxide test in gases)—Use oxalated or defibrinated blood of dogs, sheep, cattle, or human beings within 24 hours after bleeding. Prepare oxalated blood by adding 10 mg of sodium oxalate to each mL of the freshly drawn blood.
Blood Group A1 Red Blood Cells and Blood Group B Red Blood Cells—
These cells must be obtained from manufacturers or suppliers licensed by the Center for Biologics Evaluation and Research/Food and Drug Administration. The use of reagents from an unlicensed manufacturer or supplier may invalidate the results. Generally, they are available as part of a kit for ABO Blood Group testing. The cells licensed for use in test tubes can also be used in the microtiter plate method described in the monographs of
Red Blood Cells and
Whole Blood.
[NOTE—There are many manufacturers and suppliers of these reagents that are licensed by the Center for Biologics Evaluation and Research Food and Drug Administration. Some examples of licensed manufacturers or suppliers are the following: Gamma Biologics, Houston, TX; and Ortho Diagnostics, Raritan, NJ.]
Anti-A Blood Grouping Reagent, Anti-B Blood Grouping Reagent, and Anti-AB Blood Grouping Reagent—
The reagents can be monoclonal or polyclonal and must be obtained from manufacturers or suppliers licensed by the Center for Biologics Evaluation and Research, Food and Drug Administration for use in microplate tests. The use of reagents from an unlicensed manufacturer or supplier may invalidate the results. Generally, all three reagents are available as part of a kit.
[NOTE—There are many manufacturers and suppliers of these reagents that are licensed by the Center for Biologics Evaluation and Research, Food and Drug Administration. Some examples of licensed manufacturers or suppliers are the following: Gamma Biologics, Houston, TX; and Ortho Diagnostics, Raritan, NJ.]
Blue Tetrazolium
(
3,
3¢-(
3,
3¢-Dimethoxy[
1,
1¢-biphenyl]
-4,
4¢-diyl)
bis[
2,
5-diphenyl-2H-tetrazolium]
dichloride),

C
40H
32Cl
2N
8O
2—
727.64—Lemon-yellow crystals. Slightly soluble in water; freely soluble in chloroform and in methanol; insoluble in acetone and in ether.
Solubility in methanol—
Dissolve 1 g in 100 mL of methanol: complete solution results, and the solution is clear.
Color—
Transfer a portion of the methanol solution obtained in the preceding test to a 1-cm cell, and determine its absorbance at 525 nm, against water as the blank: the absorbance does not exceed 0.20.
Suitability test—
 STANDARD PREPARATION—
STANDARD PREPARATION—
Dissolve in alcohol a suitable quantity of
USP Hydrocortisone RS, previously dried at 105

for 3 hours and accurately weighed, and prepare by stepwise dilution a solution containing about 10 µg per mL.
 PROCEDURE—
PROCEDURE—
Pipet 10-, 15-, and 20-mL portions of
Standard Preparation into separate, glass-stoppered, 50-mL conical flasks. Add 10 mL and 5 mL, respectively, of alcohol to the flasks containing the 10- and 15-mL portions of
Standard Preparation, and swirl to mix. To each of the flasks, and to a fourth flask containing 20 mL of alcohol, add 2.0 mL of a solution prepared by dissolving 50 mg of blue tetrazolium in 10 mL of alcohol, mix, and then add 2.0 mL of a solution prepared by diluting 1 mL of
tetramethylammonium hydroxide TS with alcohol to 10 mL. Mix, allow the flasks to stand in the dark for 90 minutes, and determine the absorbances of the three solutions of the steroid standard at 525 nm, with a suitable spectrophotometer, using the solution in the fourth flask as the blank. Plot the absorbances on the abscissa and the amount of hydrocortisone on the ordinate scale of arithmetic coordinate paper, and draw the curve of best fit: the absorbance of each solution is proportional to the concentration, and the absorbance of the solution containing 200 µg of hydrocortisone is not less than 0.50.
Boric Acid,

H
3BO
3—
61.83—Use ACS reagent grade.
(–)-Bornyl Acetate
(1,
7,
7-Trimethylbicyclo[
2,
2,
1]-
heptan-2-ol acetate),

C
12H
20O
2—
196.29


[
5655-61-8]—Use a suitable grade.
Boron Trifluoride,

BF
3—
67.81—Use a suitable grade.
14% Boron Trifluoride–Methanol
—Use a suitable grade.
Bovine Collagen—
Use a suitable grade that contains less than 1 µg glycosaminoglycan per mg.
7 Percent Bovine Serum Albumin Certified Standard
—Available from the National Institute of Standards and Technology, www.nist.gov, as SRM 927.
Branched Polymeric Sucrose—about 400 kDa



[
26873-85-8]—White to off-white powder. Synthetic polymer made by the copolymerization of sucrose and epicholrohydrin. Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Ficoll” from Pharmacia Fine Chemicals, Inc., 800 Centennial Ave., Piscataway, NJ 08854.]
Brilliant Green
(
Malachite Green G),

C
27H
34N
2O
4S—
482.64—Glistening, golden-yellow crystals. Soluble in water and in alcohol. Absorption maximum: 623 nm.
Bromelain—
A proteolytic enzyme isolated from pineapple. Use a suitable grade.
Bromine,

Br—
At. Wt. 79.904—Use ACS reagent grade.
 -Bromo-2¢-acetonaphthone
-Bromo-2¢-acetonaphthone
(
Bromomethyl 2-naphthyl ketone),

C
12H
9BrO—
249.10—Tannish–pink crystals.
p-Bromoaniline,

C
6H
6BrN—
172.02—White to off-white crystals. Insoluble in water; soluble in alcohol and in ether.
Assay—
Transfer about 650 mg, accurately weighed, to a suitable container, and dissolve in 50 mL of
glacial acetic acid TS. Add
crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 17.20 mg of C
6H
6BrN. Not less than 98% is found.
Bromofluoromethane—
Use a suitable grade.
Change to read:
N-Bromosuccinimide,

C
4H
4BrNO
2—
177.98—White to off-white crystals or powder.

 USP29
USP29 Freely soluble in water, acetone, and glacial acetic acid.
[Caution—Highly irritating to eyes, skin, and mucous membranes.
]
Assay—
Transfer 200 mg, accurately weighed, to a conical flask, add 25 mL of 0.5 N alcoholic potassium hydroxide, cover with a watch glass, heat to boiling, and boil for 5 minutes. Cool, transfer the solution to a beaker, rinsing the flask with water until the total volume of solution plus rinsings is about 100 mL, and add 10 mL of glacial acetic acid. Insert suitable electrodes, and titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically. Each mL of 0.1 N silver nitrate is equivalent to 17.80 mg of C4H4BrNO2. Not less than 98% is found.
Brucine Sulfate,

(C
23H
26N
2O
4)
2·H
2SO
4·7H
2O—
1013.11—Use ACS reagent grade.
Buffers
—See Buffer Solutions under Solutions.
1-Butaneboronic Acid,

C
4H
11BO
2—
101.94—White flakes.
Assay—
Dissolve about 50 mg, accurately weighed, in 2 mL of bis(trimethylsilyl)acetamide, and mix while heating to boiling. Allow the solution to cool, and inject an appropriate specimen into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 250

; the detector temperature is maintained at 300

; the column temperature is maintained at 100

and programmed to rise 10

per minute to 250

. The area of the C
4H
11BO
2 peak is not less than 99% of the total peak area.
Solubility—
A solution of 50 mg in 1 mL of alcohol is clear and colorless.
1,3-Butanediol
(
1,
3-Butylene Glycol),

C
4H
10O
2—
90.12—Viscous, colorless liquid. Very hygroscopic. Soluble in water, in alcohol, in acetone, and in methyl ethyl ketone; practically insoluble in aliphatic hydrocarbons, in benzene, and in toluene.
Assay—
Inject an appropriate specimen into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 3-mm × 1.8-m stainless steel column containing 20% phase G16 on support S1A; the injection port temperature is maintained at 265

; the column temperature is maintained at 150

and programmed to rise 8

per minute to 210

. The area of the butanediol peak is not less than 98% of the total peak area.
Change to read:
2,3-Butanedione
(
Diacetyl),

CH
3COCOCH
3—
86.09—Bright yellow to yellowish-green liquid.

 USP29
USP29 Soluble in water. Miscible with alcohol and with ether. Boils at about 88

.
Assay—
HYDROXYLAMINE HYDROCHLORIDE SOLUTION—
Dissolve 20 g of hydroxylamine hydrochloride in 40 mL of water, and dilute with alcohol to 400 mL. Add, with stirring, 300 mL of 0.5 N alcoholic potassium hydroxide, and filter. Discard after 2 days.
PROCEDURE—
Transfer about 1 g, accurately weighed, to a glass-stoppered, 250-mL flask, add 75.0 mL of
Hydroxylamine hydrochloride solution, and insert the stopper in the flask. Reflux the mixture for 1 hour, then cool to room temperature. Add
bromophenol blue TS, and titrate with 0.5 N hydrochloric acid VS to a greenish-yellow endpoint.
[NOTE—Alternatively, the solution may be titrated potentiometrically to a pH of 3.4.
] Perform a blank test with the same quantities of reagent used for the test specimen, and make any necessary correction. Each mL of 0.5 N hydrochloric acid is equivalent to 43.05 mg of CH
3COCOCH
3. Not less than 97% of CH
3COCOCH
3 is found.
Butyl Acetate, Normal,

CH
3COO(CH
2)
3CH
3—
116.16—Use ACS reagent grade.
Butyl Alcohol
(1-Butanol; Normal Butyl Alcohol),

CH
3(CH
2)
2CH
2OH—
74.12—Use ACS reagent grade.
Butyl Alcohol, Secondary
(
2-Butanol),

CH
3CH
2CH(OH)CH
3—
74.12—Use ACS reagent grade Isobutyl Alcohol.
Butyl Alcohol, Tertiary,

(CH
3)
3COH—
74.12—Use ACS reagent grade
tert-butyl alcohol.
4-(Butylamino)benzoic Acid,

C
11H
5NO
2—
193.25


[
4740-24-3]—Use a suitable grade.
[NOTE—A suitable grade is available from Sigma-Aldrich, Inc., P.O. Box 2060, Milwaukee, WI 53201;
www.sigma-aldrich.com.
]
Butyl Benzoate,

C
11H
14O
2—
178.23—Thick, oily, colorless to pale yellow liquid. Practically insoluble in water; soluble in alcohol and in ether.
Assay—
When examined by gas-liquid chromatography, it shows a purity of not less than 98%. The following conditions have been found suitable for assaying it: use a 3-mm × 1.8-m stainless steel column packed with liquid phase G4 on support S1A. Helium is the carrier gas, the injection port temperature is maintained at 180

, the column temperature is 190

, and the flame-ionization detector is maintained at 280

. The retention time is about 15 minutes.
Change to read:
n-Butyl Chloride
(
1-Chlorobutane),

C
4H
9Cl—
92.57—Clear, colorless, volatile liquid.

 USP29 Highly flammable
USP29 Highly flammable. Practically insoluble in water. Miscible with alcohol and with ether.
Assay—
When examined by gas–liquid chromatography, it shows a purity of not less than 98%. The following conditions have been found suitable for assaying the article: a 3-mm × 1.8-m stainless steel column packed with phase G16 on support S1. Helium, flowing at a rate of about 40 mL per minute, is the carrier gas, the detector temperature is about 310

, the injection port temperature is about 230

, and the column temperature is programmed to rise at 10

per minute from 35

to 150

. A flame-ionization detector is employed.
Acidity—
Add
phenolphthalein TS to 75 mL, and titrate with 0.1 N potassium hydroxide in methanol to a faint pink color that persists, with shaking, for 1.5 seconds: not more than 0.91 mL is required (about 0.005% as HCl).
Water  921
921 :
:
not more than 0.02%, determined by the
Titrimetric Method.
Residue after evaporation—
Evaporate about 60 mL (50 g), accurately weighed, in a tared platinum dish on a steam bath, and dry at 105

for 1 hour: not more than 0.005% is found.
Butyl Ether
(
n-Dibutyl Ether),

C
8H
18O—
130.23—Use a suitable grade.
tert-Butyl Methyl Ether,

C
5H
12O—
88.15—Colorless liquid.
Assay—
Inject an appropriate specimen into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 100

; the detector temperature is maintained at 300

; and the column temperature is maintained at ambient temperature and programmed to rise 10

per minute to 150

. The area of the C
5H
12O peak is not less than 99.8% of the total peak area.
n-Butylamine,

CH
3CH
2CH
2CH
2NH
2—
73.14—Colorless to pale yellow, flammable liquid. Miscible with water, with alcohol, and with ether. Store it in tight containers. Specific gravity: about 0.740.
Chloride (Reagent test)—
One g (1.5 mL) shows not more than 0.01 mg of Cl (0.001%).
Acidic impurities—
To 50 mL add 5 drops of a saturated solution of azo violet in benzene, and titrate quickly with 0.1 N sodium methoxide VS to a deep blue endpoint, observing precautions to prevent absorption of atmospheric carbon dioxide as by use of an atmosphere of nitrogen: not more than 1.0 mL of 0.1 N sodium methoxide is required for neutralization.
tert-Butylamine,

C
3H
9CNH
2—
73.14—Liquid.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 230

; the detector temperature is maintained at 300

; the column temperature is maintained at 130

and programmed to rise 10

per minute to 280

. The area of the C
3H
9CNH
2 peak is not less than 99.5% of the total peak area.
n-Butylboronic Acid,

C
4H
9B(OH)
2—
101.94—Use a suitable grade.
[NOTE—This reagent is usually shipped and stored under water. Before use, remove any excess water by light vacuum filtration. A suitable grade is available from Sigma-Aldrich,
www.sigma-aldrich.com.
]
tert-Butyldimethylchlorosilane in N-Methyl-N-tert-butyldimethylsilyltrifluoroacetamide, (1 in 100)
—Use a suitable grade.
[NOTE—A suitable grade is available as 99% MTBSTFA, 1% TBDMCS from Regis Chemical Company, 8210 Austin Ave., P.O. Box 519, Morton Grove, IL 60053.]
4-tert-Butylphenol,

C
10H
14O—
150.22—White, crystalline flakes or needles. Practically insoluble in water; soluble in alcohol and in ether.
Butyric Acid,

C
4H
8O
2—
88.11—Clear, colorless to faint yellow liquid. Miscible with water and with methanol.
Assay—
Weigh accurately about 500 mg, transfer to a suitable container, add 30 mL of water, and mix. Add 40 mL of water, and mix. Add
phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sodium hydroxide is equivalent to 8.81 mg of C
4H
8O
2: not less than 99.0% of C
4H
8O
2 is found.
Butyrolactone
(Dihydro-2-(3H)-furanone, 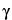 -butyrolactone)
-butyrolactone)
—
86.1—Clear, colorless to practically colorless, oily liquid. Miscible with water. Soluble in methanol and in ether.

