Change to read:
Cadmium Acetate,

C
4H
6CdO
4·2H
2O—
266.53—Colorless, transparent to translucent crystals.

 USP29
USP29 Freely soluble in water; soluble in alcohol.
Insoluble matter (Reagent test):
not more than 1 mg, from 20 g (0.005%).
Chloride (Reagent test)—
One g shows not more than 0.01 mg of Cl (0.001%).
Sulfate (Reagent test, Method II )—
Dissolve 10 g in 100 mL of water, add 1 mL of hydrochloric acid, and filter: the residue weighs not more than 1.2 mg more than the residue obtained in a complete blank test (0.005%).
Substances not precipitated by hydrogen sulfide—
Dissolve 2 g in a mixture of 135 mL of water and 15 mL of 1 N sulfuric acid, heat to boiling, and pass a rapid stream of hydrogen sulfide through the solution as it cools. Filter, and to 75 mL of the clear filtrate add 0.25 mL of sulfuric acid, then evaporate to dryness, and ignite gently: the residue weighs not more than 1 mg (0.1%).
Cadmium Nitrate,

Cd(NO
3)
2·4H
2O—
308.48—Colorless, hygroscopic crystals. Very soluble in water; soluble in alcohol.
Insoluble matter (Reagent test):

not more than 1 mg, from 20 g (0.005%).
Chloride (Cl ) (Reagent test)—
One g shows not more than 0.01 mg of Cl (0.001%).
Sulfate (Reagent test, Method II )—
Evaporate a mixture of 12 g of specimen and 25 mL of hydrochloric acid on a steam bath to dryness. Add another 15 mL of hydrochloric acid, and again evaporate to dryness. Dissolve the residue in 100 mL of water, filter, and add 1 mL of hydrochloric acid: the residue weighs not more than 1.0 mg more than the residue obtained in a blank test (0.003%).
Copper (Cu)—
Dissolve 0.5 g in 10 mL of water, add 10 mL of
Ammonium Citrate Solution (see
Lead  251
251
), and adjust the reaction of a pH of about 9 by the addition of 1 N ammonium hydroxide (about 30 mL). Add 1 mL of sodium diethyldithiocarbamate solution (1 in 1000), and mix. Add 5 mL of amyl alcohol, shake for about 1 minute, and allow the layers to separate: any yellow color in the amyl alcohol layer is not darker than that of a blank to which 0.01 mg of Cu has been added (0.002%).
Iron (Fe)—
Dissolve 1 g in 15 mL of water, add 2 mL of hydrochloric acid, and boil for 2 minutes. Cool, and add about 30 mg of ammonium persulfate and 15 mL of a solution of potassium thiocyanate in normal butyl alcohol (made by dissolving 10 g of potassium thiocyanate in 10 mL of water, warming the solution to about 30

, diluting with normal butyl alcohol to 100 mL, and shaking until clear). Shake vigorously for 30 seconds, and allow the layers to separate: any red color in the clear alcoholic layer is not darker than that of a blank to which 0.01 mg of Fe has been added (0.001%).
Lead (Pb)—
Dissolve 1.0 g in 10 mL of water, add 0.2 mL of glacial acetic acid, and filter if necessary. To a 7-mL portion of water add 0.2 mL of glacial acetic acid and 3 mL of
Standard Lead Solution (see
Lead  251
251
), and mix, to provide a blank. Then add to each solution 1.0 mL of potassium chromate solution (1 in 10), and mix: after 5 minutes, the test solution is not more turbid than the blank (0.003%).
Substances not precipitated by hydrogen sulfide—
Dissolve 2 g in 145 mL of water, add 5 mL of sulfuric acid (1 in 10), heat to boiling, and pass a rapid stream of hydrogen sulfide through the solution as it cools. Filter, and to 75 mL of the clear filtrate add 0.25 mL of sulfuric acid, then evaporate to dryness, and ignite gently: the residue weighs not more than 1 mg (0.1%).
Calcium Acetate,

Ca(C
2H
3O
2)
2·H
2O—
176.18—White, crystalline granules or powder. Soluble in about 3 parts of water; slightly soluble in alcohol. Use ACS reagent grade.
Calcium Carbonate,

CaCO
3—
100.09—Use ACS reagent grade.
[NOTE—Calcium Carbonate of a quality suitable as a primary standard is available from the National Institute of Standards and Technology, Office of Standard Reference Materials, www.nist.gov, as standard sample No. 915.]
Calcium Carbonate, Chelometric Standard,

CaCO
3—
100.09—Use ACS reagent grade.
Calcium Caseinate
—White or slightly yellow, nearly odorless, powder. Insoluble in cold water, but forms a milky solution when suspended in water, stirred, and heated.
Residue on ignition (Reagent test)—
Ignite 5 g at 550

: the residue weighs between 150 and 300 mg (3.0% to 6.0%).
Calcium—
Treat the residue from the preceding test with 10 mL of diluted hydrochloric acid, filter, and to the clear filtrate add 5 mL of
ammonium oxalate TS: it shows a white precipitate upon standing.
Loss on drying  731
731 —
—
Dry it in vacuum at 70

to constant weight: it loses not more than 7.0% of its weight.
Fat—
Suspend 1.0 g in 5 mL of alcohol in a Mojonnier flask, add 0.8 mL of stronger ammonia water and 9 mL of water, and shake. Add a second 5-mL portion of alcohol, then add successive portions of 25 mL each of ether and solvent hexane, shaking after each addition by inverting the flask 30 times. Centrifuge, decant the solvent layer, evaporate it at a low temperature, and dry on a steam bath: the residue weighs not more than 20 mg (2.0%).
Suspensibility in water—
Place 2 g in a beaker, and add cool water slowly with stirring to form a thin, smooth paste. Add additional water to make a total of 100 mL. Stir, and heat to 80

: a milky suspension is formed that does not settle after standing for 2 hours.
Calcium Chloride,

CaCl
2·2H
2O—
147.01—Use ACS reagent grade Calcium Chloride Dihydrate.
Calcium Chloride, Anhydrous (for drying),

CaCl
2—
110.98—Use ACS reagent grade Calcium Chloride Desiccant.
Change to read:
Calcium Citrate,

Ca
3(C
6H
5O
7)
2·4H
2O—
570.49—A white,

 USP29
USP29 crystalline powder. Slightly soluble in water; freely soluble in 3 N hydrochloric acid and in 2 N nitric acid; insoluble in alcohol. To 15 mL of hot 2 N sulfuric acid add in small portions and with stirring about 500 mg of calcium citrate. Boil the mixture for 5 minutes, and filter while hot: the cooled filtrate responds to the identification test for
Citrate  191
191
.
Assay—
Accurately weigh about 400 mg of the salt, previously dried at 150

to constant weight, and transfer to a 250-mL beaker. Dissolve the test specimen in 150 mL of water containing 2 mL of 3 N hydrochloric acid, add 15 mL of 1 N sodium hydroxide and 250 mg of hydroxy naphthol blue, and titrate with 0.05 M edetate disodium VS until the solution turns deep blue. Each mL of 0.05 M edetate disodium is equivalent to 8.307 mg of Ca
3(C
6H
5O
7)
2: between 97.5% and 101% is found.
Calcium oxide and carbonate—
Triturate 1 g of calcium citrate with 5 mL of water for 1 minute: the mixture does not turn red litmus blue. Then add 5 mL of warm 3 N hydrochloric acid: only a few isolated bubbles escape.
Hydrochloric acid-insoluble matter—
Dissolve 5 g by heating with a mixture of 10 mL of hydrochloric acid and 50 mL of water for 30 minutes: not more than 2.5 mg of insoluble residue remains (0.05%).
Loss on drying  731
731 —
—
Dry it at 150

to constant weight: it loses between 12.2% and 13.3% of its weight.
Arsenic  211
211 —
—
Proceed with 0.50 g as directed for organic compounds (6 ppm of As).
Calcium Hydroxide
—Use ACS reagent grade.
Change to read:
Calcium Lactate,

(CH
3CHOHCOO)
2Ca·5H
2O—
308.29—White

 USP29
USP29 granules or powder. Is somewhat efflorescent and at 120

becomes anhydrous. One g dissolves in 20 mL of water; practically insoluble in alcohol. Store it in tight containers.
Assay—
Accurately weigh about 500 mg, previously dried at 120

for 4 hours, transfer to a suitable container, and dissolve in 150 mL of water containing 2 mL of diluted hydrochloric acid. Add 15 mL of
sodium hydroxide TS and 300 mg of hydroxy naphthol blue indicator, and titrate with 0.05 M edetate disodium VS until the solution is deep blue. Each mL of 0.05 M edetate disodium is equivalent to 10.91 mg of C
6H
10CaO
6. Not less than 98% is found.
Loss on drying  731
731 —
—
Dry it at 120

for 4 hours: it loses between 25.0% and 30.0% of its weight.
Acidity—
Add
phenolphthalein TS to 20 mL of a 1 in 20 solution, and titrate with 0.10 N sodium hydroxide: not more than 0.50 mL is required to produce a pink color.
Heavy metals (Reagent test)—
Dissolve 1 g in 2.5 mL of diluted hydrochloric acid, dilute with water to 40 mL, and add 10 mL of
hydrogen sulfide TS: any brown color produced is not darker than that of a control containing 0.02 mg of added Pb (0.002%).
Magnesium and alkali salts—
Mix 1 g with 40 mL of water, carefully add 5 mL of hydrochloric acid, heat the solution, boil for 1 minute, and add rapidly 40 mL of
oxalic acid TS. Add immediately to the warm mixture 2 drops of
methyl red TS, then add
ammonia TS dropwise, from a buret, until the mixture is just alkaline. Cool to room temperature, transfer to a 100-mL graduated cylinder, dilute with water to 100 mL, mix, and allow to stand for 4 hours or overnight. Filter, and transfer to a platinum dish 50 mL of the clear filtrate, to which has been added 0.5 mL of sulfuric acid. Evaporate the mixture on a steam bath to a small bulk. Carefully heat over a free flame to dryness, and continue heating to complete decomposition and volatilization of ammonium salts. Finally ignite the residue at 800 ± 25

for 15 minutes: the residue weighs not more than 5 mg (1%).
Volatile fatty acid—
Stir about 500 mg with 1 mL of sulfuric acid, and warm: the mixture does not emit an odor of volatile fatty acid.
Calcium Nitrate,

Ca(NO
3)
2·4H
2O—
236.15—Use ACS reagent grade.
Calcium Pantothenate, Dextro
—Use Calcium Pantothenate (USP monograph).
Calcium Sulfate,

CaSO
4·2H
2O—
172.17—Use ACS reagent grade.
Calf Thymus DNA
—Use a suitable grade.
dl-10-Camphorsulfonic Acid,

C
10H
16O
4S—
232.30—White to off-white, crystals or powder. Is optically inactive.
dl-Camphene,

C
10H
16—
136.24 


[
79-92-5]—Use a suitable grade.
[NOTE—A suitable grade is available as camphene, 95%, catalog number 45,606-5, from Sigma-Aldrich,
www.sigma-aldrich.com.]
Canada Balsam



[
8007-47-4]—A natural product derived from the resin of
Abies balsamea. Use a suitable grade.
Capric Acid
(Decanoic Acid),

C
10H
20O
2—
172.26—White, solidified melt or fragments. Soluble in alcohol, in chloroform, and in ether; practically insoluble in water.
Assay—
Inject an appropriate sample dissolved in acetone into a gas chromatograph (see
Chromatography  621
621
) that is equipped with a flame-ionization detector and contains a 0.53-mm × 30-m capillary column coated with a layer of phase G25. The carrier gas is helium, flowing at a rate of 9 mL per minute. The chromatograph is programmed as follows. Initially the column temperature is equilibrated at 150

, then the temperature is increased at a rate of 10

per minute to 250

. The injection port temperature is maintained at 240

, and the detector temperature at 265

. The area of the capric acid peak is not less than 98.5% of the toal peak area.
Carbazole,

C
12H
9N—
167.21—Off-white to tan powder.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 280

; the detector temperature is maintained at 300

; and the column temperature is maintained at 280

. The area of the C
12H
9N peak is not less than 95.5% of the total peak area.
Carbon Dioxide Detector Tube
—A fuse-sealed glass tube so designed that gas may be passed through it. Contains suitable absorbing filters and support media for the indicators hydrazine and crystal violet.
Measuring range:
0.01 to 0.3 Vol.–%.
Carbon Disulfide, CS
—Use ACS reagent grade.
Carbon Disulfide, Chromatographic
—Use a suitable grade.
Carbon Monoxide Detector Tube
—A fuse-sealed glass tube so designed that gas may be passed through it. Contains suitable absorbing filters and support media for the indicators iodine pentoxide and selenium dioxide and fuming sulfuric acid.
Measuring range:
5 to 150 ppm.
Carbon Tetrachloride,

CCl
4—
153.82—Use a grade meeting the specifications of ACS
Reagent Chemicals, 8th Edition.
Carboxymethoxylamine Hemihydrochloride,

2(C
2H
5NO
3)·HCl—
218.59—White crystalline powder. Use a suitable grade.
Carmine
(
Alum Lake of Carminic Acid),

C
22H
20O
13·
xAl—



[
1390-65-4]—Red powder. Use a suitable grade.
(R )-(–)-Carvone
(
2-Methyl-5-(
1-methylethenyl)
-2-cyclohexene-1-one),

C
10H
14O—
150.22


[
6485-40-1]—Use a suitable grade.
Change to read:
Casein
—White or slightly yellow,

 USP29
USP29 granular powder. Insoluble in water and in other neutral solvents; readily dissolved by ammonia TS and by solutions of alkali hydroxides, usually forming a cloudy solution.
Residue on ignition (Reagent test)—
Ignite 2 g: the residue weighs not more than 20 mg (1.0%).
Loss on drying  731
731 —
—
Dry it at 105

to constant weight: it loses not more than 10.0% of its weight.
Alkalinity—
Shake 1 g with 20 mL of water for 10 minutes, and filter: the filtrate is not alkaline to red litmus paper.
Soluble substances—
When the filtrate from the
Alkalinity test is evaporated and dried at 105

, the residue weighs not more than 1 mg (0.1%).
Fats—
Dissolve 1 g in a mixture of 10 mL of water and 5 mL of
alcoholic ammonia TS, and shake out with two 20-mL portions of solvent hexane. Evaporate the hexane at a low temperature, and dry at 80

: the weight of the residue does not exceed 5 mg (0.5%).
Nitrogen content, Method I  461
461 :
:
between 15.2% and 16.0% of N is found, on the anhydrous basis.
Where vitamin-free casein is required, use casein that has been rendered free from the fat-soluble vitamins by continuous extraction with hot alcohol for 48 hours followed by air-drying to remove the solvent.
Catechol
(
o-Dihydroxybenzene),

C
6H
4(OH)
2—
110.11—White crystals, which become discolored on exposure to air and light. Readily soluble in water, in alcohol, in benzene, in ether, in chloroform, and in pyridine, forming clear solutions.
Residue on ignition (Reagent test)—
Ignite 500 mg with 5 drops of sulfuric acid: the residue weighs not more than 1 mg (0.2%).
Cation-Exchange Resin
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Dowex 50-W- X8-100”, from Sigma-Aldrich,
www.sigma-aldrich.com.
]
Cation-Exchange Resin, Carboxylate (Sodium Form) (50- to 100-mesh)
—Use a suitable grade.
[NOTE—A suitable grade is available as “Bio-Rex 70” from BioRad Laboratories,
www.bio-rad.com.
]
Cation-Exchange Resin, Polystyrene
—Use a suitable grade.
Cation-Exchange Resin, Styrene-Divinylbenzene
—A strongly acidic, cross-linked sulfonated resin containing about 2% of divinylbenzene. It consists of white to light tan-colored beads which may be relatively free flowing. It is available in the hydrogen form in the 25- to 50-, 45- to 100-, and 80- to 270-mesh sizes. It can be regenerated to the hydrogen form by treating with a hydrochloric acid solution (5 in 100). For satisfactory regeneration a contact time of at least 30 minutes is required after which it must be washed free of excess acid. It is insoluble in water, methanol, and acetonitrile. Suitable for use in column chromatography.
Moisture content of fully regenerated and expanded resin—
Transfer 10 to 12 mL of the resin (as received) to a flask, and convert it completely to the hydrogen form by stirring with 150 mL of hydrochloric acid solution (5 in 100) for not less than 30 minutes. Decant the acid, and wash the resin in the same manner with water until the wash water is neutral to litmus (pH 3.5).
Transfer 5 to 7 mL of the regenerated resin to a glass filtering crucible, and remove only the excess surface water by very careful suction filtration. Transfer the conditioned resin to a tared weighing bottle, and weigh. Dry in a vacuum oven at a pressure of 50 mm of mercury at 100

to 105

for 16 hours. Transfer from the vacuum oven to a desiccator, and cool to room temperature. Again weigh. The loss in weight is between 75% and 83%.
Total wet volume capacity—
Transfer 3 to 5 mL of the regenerated, undried (see
Moisture content above) resin to a 5-mL graduated cylinder, and fill it with water. Remove any air bubbles from the resin bed with a stainless steel wire, and settle the resin to its minimum volume by tapping the graduated cylinder. Record the volume of the resin.
Transfer the resin to a 400-mL beaker. Add about 5 g of sodium chloride, and titrate, stirring well, with 0.1 N sodium hydroxide to the blue end-point of bromothymol blue (pH 7.0).
(net mL NaOH × N) / (mL of resin) = mEq / mL
The total wet volume capacity of the resin is more than 0.6 mEq per mL.
Wet screen analysis—
The purpose of this test is to properly identify the mesh size of the resin. To obtain an accurate screen analysis would require special apparatus and technique.
Add 150 mL of resin to 200 mL of water in an appropriate bottle, and allow it to stand at least 4 hours to completely swell the resin.
Transfer by means of a graduated cylinder 100 mL of settled and completely swollen resin to the top screen of a series of the designated U. S. Standard 20.3-cm brass screens. Thoroughly wash the resin on each screen with a stream of water until the resin is completely classified, collecting the wash water in a suitable container. Wash the beads remaining on the respective screens back into the 100-mL graduate, and record the volume of settled resin on each screen. At least 70% of the resin will be within the specific mesh size.
Cation-Exchange Resin, Styrene-Divinylbenzene, Strongly Acidic
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Dowex 50-W- X8-100”, from Sigma-Aldrich,
www.sigma-aldrich.com.
]
Cation-Exchange Resin, Sulfonic Acid
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Amberlyst 15” or as “Dowex 50-W-X2” from Sigma-Aldrich,
www.sigma-aldrich.com.
]
Cedar Oil
(for clearing microscopic sections)—A selected, distilled oil from the wood of the red cedar,
Juniperus virginiana Linné (Fam. Pinaceae), should be used for this purpose. Refractive index: about 1.504 at 20

. For use with homogeneous immersion lenses, a specially prepared oil having a refractive index of 1.5150 ± 0.0002 at 20

is required.
Cellulose, Chromatographic
—Use a suitable grade.
Cellulose, Microcrystalline
—Use Cellulose, Microcrystalline, FCC.
Cellulose Mixture, Chromatographic
—Use a suitable grade.
[NOTE—A suitable grade is available commercially, in precoated plate form, with fluorescent indicator, from EMD Chemicals,
www.emdchemicals.com.
]
Ceric Ammonium Nitrate,

Ce(NO
3)
4·2NH
4NO
3—
548.22—Use ACS reagent grade.
Ceric Ammonium Sulfate,

Ce(SO
4)
2·2(NH
4)
2SO
4·2H
2O—
632.55—Yellow to yellowish–orange crystals. Dissolves slowly in water, but more rapidly when mineral acids are present. Use ACS reagent grade.
Ceric Sulfate,

Ce(SO
4)
2 with a variable amount of water—
(anhydrous) 332.24—It may also contain sulfates of other associated rare earth elements. Yellow to orange-yellow crystals or crystalline powder. Practically insoluble in cold water; slowly soluble in cold dilute mineral acids, but more readily soluble when heated with these solvents.
Assay—
Weigh accurately about 800 mg, transfer to a flask, add 25 mL of water and 3 mL of sulfuric acid, and warm until dissolved. Cool, and add 60 mL of a mixture of 1 volume of phosphoric acid and 20 volumes of water. Add 25 mL of potassium iodide solution (1 in 10), insert the stopper in the flask, and allow to stand for 15 minutes. Replace the air over the solution with carbon dioxide, and while continuing the flow of carbon dioxide into the flask, titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of
starch TS as the endpoint is approached. Each mL of 0.1 N sodium thiosulfate is equivalent to 33.22 mg of Ce(SO
4)
2. Not less than 80.0% is found.
Chloride (Reagent test)—
Dissolve 1 g in a mixture of 5 mL of nitric acid and 4 mL of water. Filter, if necessary, and dilute with water to 20 mL. To 10 mL of the dilution add 1 mL of
silver nitrate TS, allow to stand for 10 minutes, and filter until clear. To the remaining 10 mL of test solution add 1 mL of
silver nitrate TS: any turbidity produced does not exceed that in a control prepared by adding 0.05 mg of Cl to the filtrate obtained from the first 10 mL of test solution (0.01%).
Heavy metals—
Heat 500 mg with a mixture of 10 mL of water and 0.5 mL of sulfuric acid until solution is complete. Cool, dilute with water to 50 mL, and bubble hydrogen sulfide gas through the solution until it is saturated: the precipitate that is formed is white or not darker than pale yellow.
Iron—
Dissolve 100 mg in a mixture of 5 mL of water and 2 mL of hydrochloric acid, warming if necessary, and cool. Transfer to a glass-stoppered cylinder, dilute with water to 25 mL, and add 5 mL of
ammonium thiocyanate TS and 25 mL of ether. Shake gently, but well, and allow the layers to separate: any pink color in the ether layer is not darker than that of a control, similarly prepared, containing 0.02 mg of added Fe (0.02%).
Cesium Chloride,

CsCl—
168.36 


[
7647-17-8]—A white powder. Very soluble in water; freely soluble in methanol; practically insoluble in acetone. Use a suitable grade.
Cetrimide
—See Cetyltrimethylammonium Bromide.
Cetyltrimethylammonium Chloride, 25 Percent in Water,

C
19H
42ClN—
320.00—Use a suitable grade.
Change to read:
Charcoal, Activated
(
Activated Carbon; Decolorizing Carbon)
—A fine, black

 USP29
USP29 powder, which is the residue from the destructive distillation of various organic materials, treated to increase its high capacity for adsorbing organic coloring substances, as well as nitrogenous bases.
Adsorptive power—
Dissolve 100 mg of strychnine sulfate in 50 mL of water, add 1 g of the test specimen, shake during 5 minutes, and pass through a dry filter, rejecting the first 10 mL of the filtrate. To a 10-mL portion of the subsequent filtrate add 1 drop of hydrochloric acid and 5 drops of mercuric iodide TS: no turbidity is produced.
Residue on ignition (Reagent test)—
Ignite 500 mg: the residue weighs not more than 20 mg (4.0%).
Reaction—
Boil 2 g with 50 mL of water for 5 minutes, allow to cool, restore the original volume by the addition of sufficient water, and filter: the filtrate is colorless and is neutral to litmus paper.
Acid-soluble substances—
Boil 1.0 g with 25 mL of dilute hydrochloric acid (1 in 5) for 5 minutes, filter into a tared porcelain crucible, and wash the residue with 10 mL of hot water, adding the washings to the filtrate. To the combined filtrate and washings add 1 mL of sulfuric acid, evaporate to dryness, and ignite to constant weight: the residue weighs not more than 35 mg (3.5%).
Alcohol-soluble substances—
Boil 2 g with 40 mL of alcohol for 5 minutes under a reflux condenser, and filter. Evaporate 20 mL of the filtrate on a steam bath, and dry at 105

for 1 hour: the residue weighs not more than 2 mg (0.2%).
Uncarbonized constituents—
To 250 mg add 10 mL of
sodium hydroxide TS, heat to boiling, and filter: the filtrate is colorless.
Chloride (Reagent test)—
A 5-mL portion of the filtrate obtained in the test for Reaction shows not more than 0.04 mg of Cl (0.02%).
Sulfate (Reagent test, Method I )—
A 5-mL portion of the filtrate from the test for Reaction shows not more than 0.3 mg of SO4 (0.15%).
Sulfide—
Place 1 g in a small flask with a narrow neck, add 35 mL of water and 5 mL of hydrochloric acid, and boil gently: the escaping vapors do not darken paper moistened with
lead acetate TS.
Chenodeoxycholic Acid,

C
24H
40O
4—
392.57—White to off-white powder.
Assay—
When tested by thin-layer chromatography, with the use of plates coated with chromatographic reversed-phase C18 mixture, a developing system consisting of 1 N acetic acid in methanol and 1 N acetic acid (19:1), and sprayed with a mixture of sulfuric acid and methanol (1:1), heated at 110

for 20 minutes, and examined visually and under long-wavelength UV light, a single spot is exhibited.
Chloramine T
(Sodium p-Toluenesulfonchloramide),

C
7H
7ClNNaO
2S·3H
2O—
281.69—Use ACS reagent grade.
Chlorine,

Cl
2—
70.9—Greenish–yellow gas. High-purity grade available from most suppliers of specialty gases.
Chlorine Detector Tube
—A fuse-sealed glass tube so designed that gas may be passed through it and containing suitable absorbing filters and support media for the indicator
o-tolidine.
Measuring range:
0.2 to 3 ppm.
m-Chloroacetanilide,

C
8H
8ClNO—
169.61—Off-white to beige granules.
Assay—
MOBILE PHASE—
Prepare a mixture of acetonitrile and water (22:3).
PROCEDURE—
Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. The area of the C
8H
8ClNO peak is not less than 99.9% of the total peak area.
p-Chloroacetanilide,

C
8H
8ClNO—
169.61—White or pale yellow, needle-shaped crystals or crystalline powder. Insoluble in water; soluble in alcohol and in ether.
Solubility—
One g dissolves in 30 mL of alcohol to form a clear solution.
Residue on ignition (Reagent test):

not more than 0.1%.
1-Chloroadamantane,

C
10H
15Cl—
170.68—White crystalline solid.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 250

; the detector temperature is maintained at 300

; and the column temperature is maintained at 150

and programmed to rise 10

per minute to 280

. The area of the C
10H
15Cl peak is not less than 97.5% of the total peak area.
3-Chloroaniline,

C
6H
6ClN—
127.57—Colorless to light brown liquid. Soluble in acid and in most organic solvents; practically insoluble in water.
Assay—
Inject an appropriate specimen into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 250

; the detector temperature is maintained at 300

; and the column temperature is maintained at 150

and programmed to rise 10

per minute to 280

. The area of the C
6H
6ClN peak is not less than 99% of the total peak area.
p-Chloroaniline,
(4-Chloroaniline),

C
6H
6ClN—
127.57


[
106-47-8]—Use a suitable grade.
Change to read:
Chlorobenzene,

C
6H
5Cl—
112.56—Clear, colorless liquid.

 USP29
USP29 Insoluble in water; soluble in alcohol, in benzene, in chloroform, and in ether. Use ACS reagent grade.
m-Chlorobenzoic Acid (3-Chlorobenzoic Acid),

C
7H
5ClO
2—
156.57—Use a suitable grade.
4-Chlorobenzoic Acid—

ClC
6H
4COOH—
156.57—White, crystalline solid.
Assay—
Dissolve about 700 mg, accurately weighed, in a mixture of 100 mL of hot alcohol and 50 mL of water. Titrate with 0.5 N sodium hydroxide VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.5 N sodium hydroxide is equivalent to 78.28 mg of ClC6H4COOH. Not less than 98% is found.
Solubility—
One g dissolved in 25 mL of 0.5 N sodium hydroxide yields a clear and complete solution.
4-Chlorobenzophenone,

C
13H
9ClO—
216.66—Use a suitable grade.
1-Chlorobutane
—See n-Butyl Chloride.
2-Chloroethylamine Monohydrochloride,

C
2H
6ClN·HCl—
115.99—Off-white powder.
Assay—
Inject an appropriate specimen into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of a phase consisting of 14% cyanopropylphenyl-86% dimethylpolysiloxane; the injection port temperature is maintained at 150

; the detector temperature is maintained at 300

; and the column temperature is maintained at 50

and programmed to rise 10

per minute to 200

. The area of the C
2H
6ClN·HCl peak is not less than 99% of the total peak area.
Chloroform,

CHCl
3—
119.38—Use ACS reagent grade.
Chloroform, Alcohol-Free
—Use a suitable grade that does not contain alcohol as a stabilizer.
Chlorogenic Acid,

C
16H
18O
9—
354.31—White to off-white powder. Use a suitable grade.
Assay—
When tested by thin-layer chromatography (see
Chromatography  621
621
) with the use of plates coated with chromatographic silica gel mixture and a developing system consisting of a mixture of butyl alcohol, water, and acetic acid (60:25:15), and examined under short-wavelength UV light, a single spot is exhibited, with trace impurities.
1-Chloronaphthalene
(
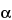 -Chloronaphthalene
-Chloronaphthalene),

C
10H
7Cl—
162.62—Colorless to light yellow liquid.
Assay—
Use a gas chromatograph equipped with a flame-ionization detector. The following conditions have been found suitable: a 3.2-mm × 1.83-m stainless steel column is packed with 7% phase G2 on support S1A; the injection port temperature is maintained at 250

and the detector at 310

; and the column temperature is programmed to increase at a rate of 10

per minute from 50

to 250

. Not less than 90% of C
10H
7Cl is found, of which not more than 10% is 2-chloronaphthalene.
4-Chloro-1-naphthol,

C
10H
7ClO—
178.6


[
604-44-4]—A white to off-white powder, with a melting point between 118

and 120

. Use a suitable grade. Store below 0

.
2-Chloronicotinic Acid,

C
6H
4ClNO
2—
157.55—Off-white powder.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 280

; the detector temperature is maintained at 300

; and the column temperature is maintained at 180

and programmed to rise 10

per minute to 280

. The area of the C
6H
4ClNO
2 peak is not less than 98% of the total peak area.
2-Chloro-4-nitroaniline, 99%,

C
6H
5ClN
2O
2—
172.57—White to off-white powder.
Chloroplatinic Acid,

H
2PtCl
6·6H
2O—
517.90—Use ACS reagent grade Chloroplatinic Acid Hexahydrate.
5-Chlorosalicylic Acid,

C
7H
5ClO
3—
172.57—White to off-white powder.
Assay—
When tested by thin-layer chromatography (see
Chromatography  621
621
) with the use of plates coated with chromatographic silica gel mixture and a developing system consisting of a mixture of cyclohexane, chloroform, and acetic acid (14:4:2), and examined visually and under long-wavelength UV light or iodine spray, a single spot is exhibited.
1-Chloro-2,2,2-trifluoroethylchlorodifluoromethyl Ether
—Use a suitable grade.
Chlorotrimethylsilane,

C
3H
9ClSi—
108.64—Clear, colorless to light yellow liquid. Fumes when exposed to moist air.
Caution—It reacts vigorously with water, alcohols, and other hydrogen donors. Store in tight glass containers.
Chlortetracycline Hydrochloride
—Use Chlortetracycline Hydrochloride (USP monograph).
Cholestane,

C
27H
48—
372.67—Use a suitable grade.
Cholesterol,

C
27H
46O—
386.66


[
57-88-5]—Use a suitable grade (
NF monograph).
Cholesteryl Benzoate,

C
34H
50O
2—
490.76—Use a suitable grade.
Cholesteryl n-Heptylate
—Use a suitable grade.
Choline Chloride,

HOCH
2CH
2N(CH
3)
3Cl—
139.62—White crystals or crystalline powder. Very soluble in water. Is hygroscopic. Store in tight containers.
Assay—
Transfer about 100 mg, previously dried at 105

for 2 hours and accurately weighed, to a beaker, add 20 mL of water and 1 drop of aluminum chloride solution (1 in 10), and mix. Add, slowly, 20 mL of a freshly prepared, filtered sodium tetraphenylborate solution (1 in 50), and allow the mixture to stand for 30 minutes with occasional swirling. Filter through a medium-porosity, sintered-glass filter, and wash the beaker and the precipitate with four 10-mL portions of water. The weight of the precipitate, determined after drying at 105

for 2 hours, and multiplied by 0.3298, gives the equivalent weight of C
5H
14ClNO. Not less than 99.5% is found.
Chromatographic n-Heptane
—See n-Heptane, Chromatographic.
Chromatographic Reagents—
See
Chromatography  621
621
.
[NOTE—Listings of the numerical designations for phases (G), packings (L), and supports (S), together with corresponding brand names, are published periodically in Pharmacopeial Forum as a guide for the chromatographer.]
Chromatographic Solvent Hexane
—See Hexane, Solvent, Chromatographic.
Chromium Potassium Sulfate Dodecahydrate,

CrK(SO
4)
2·12H
2O—
499.40


[
10279-63-7]—Use ACS reagent grade.
Chromium Trioxide,

CrO
3—
99.99—Use ACS reagent grade.
Chromogenic Substrate for Amidolytic Test
—Synthetic molecules consisting of tripeptides or tetrapeptides coupled to a chromophore. The terminal amino acid is specific for the protease utilized. The synthetic peptides mimic the peptide sequence (specific to the activated coagulation factor) of the active site on the natural substrate. The coagulation factor catalyzes the splitting of the chromophore (
p-nitroaniline) from the peptide. The amount of release can be measured directly in a spectrophotometer, because the maximum absorbance spectra of the bound and free chromophores differ. The released chromophore is a colored compound; the complete substrate itself is colorless.
Molecular weights of the various substrates range from about 600 to 750 Da. Solubility in aqueous solutions can vary. Not all substrates are of equal sensitivity, and incubation periods may have to be extended.
Chromotrope 2R,

C
16H
10N
2Na
2O
8S
2—
468.4


[
4197-07-3]—Red powder or crystals. Use a suitable grade.
Chromotropic Acid
(4,5-Dihydroxy-2,7-naphthalenedisulfonic Acid),

C
10H
8O
8S
2·2H
2O—
356.33—Use a suitable grade.
Chromotropic Acid Disodium Salt
(
4,
5-Dihydroxy-2,
7-naphthalenedisulfonic Acid,
Disodium Salt),

C
10H
6O
8Na
2S
2·2H
2O—
400.29—Use ACS reagent grade.
Cinchonidine,

C
19H
22N
2O—
294.39—White crystals, crystalline or granular powder. Soluble in alcohol and in chloroform; practically insoluble in water.
Assay—
Dissolve about 125 mg, accurately weighed, in 50 mL of glacial acetic acid. Add a few drops of p-naphtholbenzein TS and titrate with 0.1 N perchloric acid VS. Perform a blank determination and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 14.72 mg of C19H22N2O. Not less than 99.0% is found.
Loss on drying  731
731 —
—
Dry it at 105

to constant weight: it loses not more than 1.0% of its weight.
Specific rotation  781
781 :
:

between
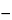
105

and
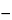
115

, calculated on the dried basis, determined in a solution in alcohol containing 10 mg per mL.
Cinchonine,

C
19H
22N
2O—
294.39—White crystals, crystalline or granular powder. Slightly soluble in chloroform, sparingly soluble in alcohol and practically insoluble in water.
Assay—
Dissolve about 125 mg, accurately weighed, in 50 mL of glacial acetic acid. Add a few drops of p-naphtholbenzein TS and titrate with 0.1 N perchloric acid VS. Perform a blank determination and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 14.72 mg of C19H22N2O. Not less than 99.0% is found.
Loss on drying  731
731 —
—
Dry it at 105

to constant weight: it loses not more than 1.0% of its weight.
Cobalt Chloride
(
Cobaltous Chloride),

CoCl
2·6H
2O—
237.93—Use ACS reagent grade.
Cobalt Nitrate,

Co(NO
3)
2·6H
2O—
291.03—Use ACS reagent grade.
Cobaltous Acetate
(Cobalt Acetate),

Co(C
2H
3O
2)
2·4H
2O—
249.08—Red, needle-like crystals. Soluble in water and in alcohol. Use ACS reagent grade.
Coenzyme Q9
(
Ubiquinone 45),

C
54H
82O
4—
795.2


[
303-97-9]—Yellow to yellow-orange powder. Clear yellow solution, at a concentration of 1 mg per mL in a mixture of chloroform and ethanol (9:1).
Absorptivity—
Its absorptivity at 275 nm, in alcohol solution, is about 1700.
Collagen—
Use a suitable grade.
[NOTE—A suitable grade is acid-soluble Collagen Type I from calf skin, and is commercially available from Sigma-Aldrich Corp.,
www.sigma-aldrich.com; catalog number C3511.
]
Rat Tail Collagen
—Use a suitable grade.
Collagenase—
Use a suitable grade.
[NOTE—A suitable grade is commercially available as Collagenase Type 2, CLS-2, from Worthington Biochemical Corp.,
www.worthington-biochem.com.
]
Compactin,

C
23H
34O
5—
390.52 


[
73573-88-3]—Use a suitable grade.
Change to read:
Congo Red,

C
32H
22N
6Na
2O
6S
2—
696.67—A dark red or reddish-brown powder.

 USP29
USP29 Decomposes on exposure to acid fumes. Its solutions have a pH of about 8 to 9.5. One g dissolves in about 30 mL of water. Is slightly soluble in alcohol.
Residue on ignition—
Accurately weigh about 1 g, previously dried at 105

for 4 hours, and place it in a porcelain dish or crucible. Ignite carefully until well charred, cool, add 2 mL of sulfuric acid, and carefully ignite until the residue is white or practically so. Cool, add 0.5 mL of sulfuric acid and 1 mL of nitric acid, evaporate, and again ignite to constant weight: the weight of the sodium sulfate so obtained is between 20.0% and 24.0% of the weight of the dried specimen taken.
Sensitiveness—
To 50 mL of carbon dioxide-free water add 0.1 mL of congo red solution (1 in 1000). The red color of the solution is changed to violet by the addition of 0.05 mL of 0.10 N hydrochloric acid and is restored by the subsequent addition of 0.05 mL of 0.10 N sodium hydroxide.
Coomassie Blue G-250 (Coomassie Brilliant Blue G-250, Serva Blue G),

C
47H
48N
3O
7S
2Na—
854.0


[
6104-58-1]—A dark blue powder. Soluble in water. Use a suitable grade. Store between 15

and 30

.
Coomassie Brilliant Blue R-250,

C
45H
44N
3O
7S
2Na—
825.97—Brown powder.
Copper,

Cu—
At. Wt. 63.546—Use ACS reagent grade.
Cortisone,

C
21H
28O
5—
360.44—White, crystalline powder. Practically insoluble in water; sparingly soluble in alcohol and in acetone. Melts at about 220

, with decomposition.
Absorption maximum—
The UV absorption spectrum of a 1 in 100,000 solution in alcohol shows a maximum at about 238 nm.
Cotton, Absorbent
—Use Purified Cotton (USP monograph).
m-Cresol Purple

C
21H
18O
5S—
382.43—Use a suitable grade.
Cupric Acetate,

Cu(C
2H
3O
2)
2·H
2O—
199.65—Use ACS reagent grade.
Cupric Chloride,

CuCl
2·2H
2O—
170.48—Bluish–green deliquescent crystals. Freely soluble in water; soluble in alcohol; slightly soluble in ether. Use ACS reagent grade.
Cupric Citrate
([
Citrato(
4-)]
dicopper),

Cu
2C
6H
4O
7—
315.18—Use a suitable grade.
Cupric Nitrate—



[
3251-23-8]—Use ACS reagent grade Cupric Nitrate Hydrate.
Cupric Nitrate Hydrate,
 Cu(NO3
Cu(NO3)
2·2.5H
2O—
232.59


[
3252-23-8]; Cu(NO
3)
2·3H
2O—
241.60 [
10031-43-3]—Use ACS reagent grade.
[NOTE—This reagent is available containing either 2.5 or 3 molecules of water of hydration.]
Cupric Sulfate,

CuSO
4·5H
2O—
249.69—Use ACS reagent grade.
Cupric Sulfate, Anhydrous,

CuSO
4—
159.61—A white or grayish–white powder free from a blue tinge. Upon the addition of a small quantity of water, it becomes blue. Soluble in water. Store in tight containers.
Chloride (Reagent test)—
One g shows not more than 0.02 mg of Cl (0.002%).
Substances not precipitated by hydrogen sulfide—
Determine as directed for ACS reagent grade of Cupric Acetate: the residue weighs not more than 6 mg (0.15%).
Cupriethylenediamine Hydroxide Solution, 1.0 M
—Use a suitable grade.
Cyanoacetic Acid,

C
3H
3NO
2—
85.06—White to light yellow, crystalline solid. Very soluble in water.
Assay—
Dissolve about 300 mg, accurately weighed, in 25 mL of water and 25 mL of alcohol. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. Perform a blank determination and make any necessary corrections. Each mL of 0.1 N sodium hydroxide is equivalent to 85.06 mg of C3H3NO2. Not less than 99% is found.
Cyanogen Bromide,

BrCN—
105.92—Colorless crystals. Volatilizes at room temperature. Its vapors are highly irritating and
very toxic. Melts at about 52

. Freely soluble in water and in alcohol. Store in tight containers in a cold place.
Solubility—
Separate 1-g portions dissolve completely in 10 mL of water and in 10 mL of alcohol, respectively, to yield colorless solutions.
4-Cyanophenol
(
4-Hydroxybenzonitrile),

C
6H
4CNOH—
119.12


[
767-00-0]—Use 95 percent reagent.
Cyclam
(
1,
4,
8,
11-Tetraazacyclotetradecane),

C
10H
24N
4—
200.33


[
295-37-4]—Use 98 percent reagent.
Cyclohexane,

C
6H
12—
84.16—Use ACS reagent grade.
Change to read:
Cyclohexanol,

C
6H
12O—
100.16—A clear liquid.

 USP29
USP29 Freely soluble in water. Miscible with alcohol, with ethyl acetate, and with aromatic hydrocarbons.
Assay—
When examined by gas-liquid chromatography, using suitable gas chromatographic apparatus and conditions, it shows a purity of not less than 98%.
Melting temperature:
about 23

.
Specific gravity:
about 0.962, at 20

.
(1,2-Cyclohexylenedinitrilo)tetraacetic Acid
(
trans-1,
2-Diaminocyclohexane-N,
N,
N¢,
N¢-tetraacetic Acid),

C
14H
22N
2O
8·H
2O—
364.35—Use ACS reagent grade.
Cyclohexylmethanol,

C
7H
14O—
114.19—Use a suitable grade.
L-Cystine,

HOOC(NH
2)CHCH
2S—SCH
2CH(NH
2)COOH—
240.30—A white, crystalline powder. Very slightly soluble in water; soluble in dilute mineral acids and in solutions of alkali hydroxides; insoluble in alcohol and in other organic solvents.
Specific rotation  781
781 :
:

between
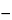
215

and
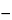
225

, determined in a 2 in 100 solution of test specimen, previously dried over silica gel for 4 hours, in dilute hydrochloric acid (1 in 10) at a temperature of 20

.
Loss on drying  731
731 —
—
Dry it over silica gel for 4 hours: it loses not more than 0.2% of its weight.
Residue on ignition (Reagent test):

not more than 0.1%.

