 161
161 TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES
TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES
The requirements apply to sterile and nonpyrogenic assemblies or devices in contact directly or indirectly with the cardiovascular system, the lymphatic system, or cerebrospinal fluid. This includes, but is not limited to, solution administration sets, extension sets, transfer sets, blood administration sets, intravenous catheters, implants extracorporeal oxygenator tubings and accessories, dialysers and dialysis tubing and accessories, heart valves, vascular grafts, intramuscular drug delivery catheters, and transfusion and infusion assemblies. These requirements do not apply to orthopedic products, latex gloves, or wound dressings.
Bacterial Endotoxins—
Proceed as directed under
Bacterial Endotoxins Test  85
85
.
For medical devices, the endotoxin limit is not more than 20.0 USP Endotoxin Units per device except that for those medical devices in contact with the cerebrospinal fluid the limit is not more than 2.15 USP Endotoxin Units per device.
A device that fails this test can be retested once by another
Bacterial Endotoxins test. For devices that cannot be tested by the
Bacterial Endotoxins Test  85
85
because of nonremovable inhibition or enhancement, the
Pyrogen Test  151
151
is applied.
Preparation of Devices—
Select not less than 3 and not more than 10 devices. Rinse or soak the devices with LAL Reagent Water. The volume of rinsing or extracting solution may be adjusted for the size and configuration of the device.
For devices labeled “nonpyrogenic fluid pathway,” flush the fluid pathway with extracting fluid that has been heated to 37 ± 1.0

, keeping the extracting fluid in contact with the relevant pathway for not less than 1 hour at controlled room temperature. Extracts may be combined, where appropriate. The endotoxin limit for the rinsing or extracting solution is calculated by the formula:
(K × N) / (V)
where
K is equal to the amount of endotoxin allowed per device,
N is equal to the number of devices tested, and
V is equal to the total volume of the extract or rinse. If the undiluted rinsing or extracting solution is unsuitable for the
Bacterial Endotoxins Test  85
85
, repeat the inhibition or enhancement test after neutralization and removal of the interfering substances or after the solution has been diluted by a factor not exceeding the Maximum Valid Dilution. The Maximum Valid Dilution for devices is calculated by dividing the endotoxin limit by the labeled sensitivity
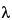
of the LAL reagent used.
Pyrogen—
For samples that cannot be tested by the
Bacterial Endotoxins Test because of nonremovable inhibition or enhancement of the test, the
Pyrogen Test  151
151
is applied. Select 10 devices, and obtain a pooled effluent, utilizing preparation methods appropriate to the device as directed for
Bacterial Endotoxins, but with volumes of rinse or extraction fluid not to exceed 40 mL of sterile saline TS per device. The requirements of the
Pyrogen Test  151
151
are met.
Auxiliary Information—
Staff Liaison :
Radhakrishna S Tirumalai, Ph.D., Scientist
Expert Committee : (GTMDB05) General Toxicology and Medical Device Biocompatibility
USP31–NF26 Page 124
Phone Number : 1-301-816-8339

