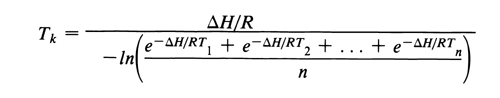The term “stability,” with respect to a drug dosage form, refers to the chemical and physical integrity of the dosage unit and, when appropriate, the ability of the dosage unit to maintain protection against microbiological contamination. The shelf life of the dosage form is the time lapse from initial preparation to the specified expiration date. The monograph specifications of identity, strength, quality, and purity apply throughout the shelf life of the product.
The stability parameters of a drug dosage form can be influenced by environmental conditions of storage (temperature, light, air, and humidity), as well as the package components. Pharmacopeial articles should include required storage conditions on their labeling. These are the conditions under which the expiration date shall apply. The storage requirements specified in the labeling for the article must be observed throughout the distribution of the article (i.e., beyond the time it leaves the manufacturer up to and including its handling by the dispenser or seller of the article to the consumer). Although labeling for the consumer should indicate proper storage conditions, it is recognized that control beyond the dispenser or seller is difficult. The beyond-use date shall be placed on the container label.
Stability Protocols
Stability of manufactured dosage forms must be demonstrated by the manufacturer, using methods adequate for the purpose. Monograph assays may be used for stability testing if they are stability-indicating (i.e., if they accurately differentiate between the intact drug molecules and their degradation products). Stability considerations should include not only the specific compendial requirements, but also changes in physical appearance of the product that would warn users that the product's continued integrity is questionable.
Stability studies on active substances and packaged dosage forms are conducted by means of “real-time,” long-term tests at specific temperatures and relative humidities representing storage conditions experienced in the distribution chain of the climatic zone(s) of the country or region of the world concerned. Labeling of the packaged active substance or dosage form should reflect the effects of temperature, relative humidity, air, and light on its stability. Label temperature storage warnings will both reflect the results of the real-time storage tests and allow for expected seasonal excursions of temperature.
Controlled Room Temperature
Controlled room temperature (see Storage Temperature and Humidity in Preservation, Packaging, Storage, and Labeling under General Notices and Requirements) delineates the allowable tolerance in storage circumstances at any location in the chain of distribution (e.g., pharmacies, hospitals, and warehouses). This terminology also allows patients or consumers to be counseled as to appropriate storage for the product. Products may be labeled either to store at “Controlled room temperature” or to store at temperatures “up to 25 ” where labeling is supported by long-term stability studies at the designated storage condition of 25
” where labeling is supported by long-term stability studies at the designated storage condition of 25 . Controlled room temperature limits the permissible excursions to those consistent with the maintenance of a mean kinetic temperature calculated to be not more than 25
. Controlled room temperature limits the permissible excursions to those consistent with the maintenance of a mean kinetic temperature calculated to be not more than 25 . See Mean Kinetic Temperature. The common international guideline for long-term stability studies specifies 25 ± 2
. See Mean Kinetic Temperature. The common international guideline for long-term stability studies specifies 25 ± 2 at 60 ± 5% relative humidity. Accelerated studies are specified at 40 ± 2
at 60 ± 5% relative humidity. Accelerated studies are specified at 40 ± 2 and at 75 ± 5% relative humidity. Accelerated studies also allow the interpretation of data and information on short-term spikes in storage conditions in addition to the excursions allowed by controlled room temperature.
and at 75 ± 5% relative humidity. Accelerated studies also allow the interpretation of data and information on short-term spikes in storage conditions in addition to the excursions allowed by controlled room temperature.
The term “room temperature” is used in different ways in different countries, and for products to be shipped outside the continental U.S. it is usually preferable for product labeling to refer to a maximum storage temperature or temperature range in degrees Celsius.
Mean Kinetic Temperature
Mean Kinetic Temperature (MKT) is defined as the single calculated temperature at which the total amount of degradation over a particular period is equal to the sum of the individual degradations that would occur at various temperatures. Thus, MKT may be considered as an isothermal storage temperature that simulates the nonisothermal effects of storage temperature variation. It is not a simple arithmetic mean. MKT is calculated from temperatures in a storage facility. The temperatures for calculating MKT can be conveniently collected using electronic devices that measure temperatures at frequent intervals (e.g., every 15 minutes). MKT can be calculated directly or the data can be downloaded to a computer for processing. For dispensing sites, such as pharmacies and hospitals, where the use of such instruments may not be feasible, devices such as high-low thermometers capable of indicating weekly high and low temperatures over a 52-week period may be employed. The arithmetic mean of the weekly high and low temperatures is then used in the calculation of MKT. MKT is calculated by the following equation (derived from the Arrhenius equation):
in which Tk is the mean kinetic temperature; DH is the heat of activation, 83.144 kJ·mole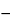 1 (unless more accurate information is available from experimental studies); R is the universal gas constant, 8.3144 × 10
1 (unless more accurate information is available from experimental studies); R is the universal gas constant, 8.3144 × 10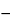 3 kJ·mole
3 kJ·mole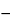 1·degree
1·degree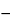 1; T1 is the value for the temperature recorded during the first time period, e.g., the first week; T2 is the value for the temperature recorded during the second time period, e.g., second week; and Tn is the value for the temperature recorded during the nth time period, e.g., nth week, n being the total number of storage temperatures recorded (minimum of 52 weekly entries) during the annual observation period. [note—All temperatures, T, are absolute temperatures in degrees Kelvin (K).]
1; T1 is the value for the temperature recorded during the first time period, e.g., the first week; T2 is the value for the temperature recorded during the second time period, e.g., second week; and Tn is the value for the temperature recorded during the nth time period, e.g., nth week, n being the total number of storage temperatures recorded (minimum of 52 weekly entries) during the annual observation period. [note—All temperatures, T, are absolute temperatures in degrees Kelvin (K).]
The following is an example of a typical storage and distribution temperature range in Kelvin degrees and the conversion factors used to convert this range into degrees Fahrenheit and Celsius.
| Kelvin (K) | Fahrenheit ( |
Celsius ( |
| 288.1–303.1 | 59–86 | 15–30 |
| Conversion Factors: | ||
| Fahrenheit to Kelvin = {[( |
||
| Celsius to Kelvin = 273.1 + |
||
| Fahrenheit to Celsius = [( |
Climatic Zones
For convenience in planning for packaging and storage, and for stability studies, international practice identifies four climatic zones, which are described in Table 1. The United States, Europe, and Japan are characterized by zones I and II. The values in Table 1 are based on observed temperatures and relative humidities, both outside and in rooms, from which mean kinetic temperatures and average humidity values are calculated.1 Derived values are based on inspection of data from individual cities and on allowances for a margin of safety in assignment of these specified conditions.
Table 1. International Climatic Zones
| Calculated Data | Derived Data | ||||||
| Climatic Zone | % RH |
mbar*** | % RH |
mbar | |||
| I. Temperate
Japan United Kingdom Northern Europe Canada Russia United States |
20.0 | 20.0 | 42 | 9.9 | 21 | 45 | 11.2 |
| II. Mediterranean, Subtropical
United States Japan Southern Europe (Portugal-Greece) |
21.6 | 22.0 | 52 | 13.5 | 25 | 60 | 19.0 |
| III. Hot, Dry
Iran Iraq Sudan |
26.4 | 27.9 | 35 | 11.9 | 30 | 35 | 15.0 |
| IV. Hot, Humid
Brazil Ghana Indonesia Nicaragua Philippines |
26.7 | 27.4 | 76 | 26.6 | 30 | 70 | 30.0 |
|
*
Data recorded as <19
|
|||||||
|
**
Calculated mean kinetic temperature.
|
|||||||
|
***
Partial pressure of water vapor.
|
|||||||
A discussion of aspects of drug product stability that are of primary concern to the pharmacist in the dispensing of medications may be found under Stability Considerations in Dispensing Practice  1191
1191 .
.
Inasmuch as this chapter is for purposes of general information only, no statement herein is intended to modify or supplant any of the specific requirements pertinent to pharmaceutical preparations, which are given elsewhere in this Pharmacopeia.
1
The source of the data and information in Table 1 is the International Conference on Harmonization sponsored by the International Federation of Pharmaceutical Manufacturers Associations.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Desmond G. Hunt, Ph.D.
Scientist 1-301-816-8341 |
(PS05) Packaging and Storage 05 |
USP32–NF27 Page 662
Pharmacopeial Forum: Volume No. 32(4) Page 1232