This test is provided to determine compliance with drug-release requirements where specified in individual monographs. Use the apparatus specified in the individual monograph. Replace the aliquots withdrawn for analysis with equal volumes of fresh Dissolution Medium at the temperature specified in the monograph or, where it can be shown that replacement of the medium is not necessary, correct for the volume change in the calculation. Keep the vessel covered for the duration of the test, and verify the temperature of the mixture under test at suitable times.
TRANSDERMAL DELIVERY SYSTEMS—GENERAL DRUG RELEASE STANDARDS
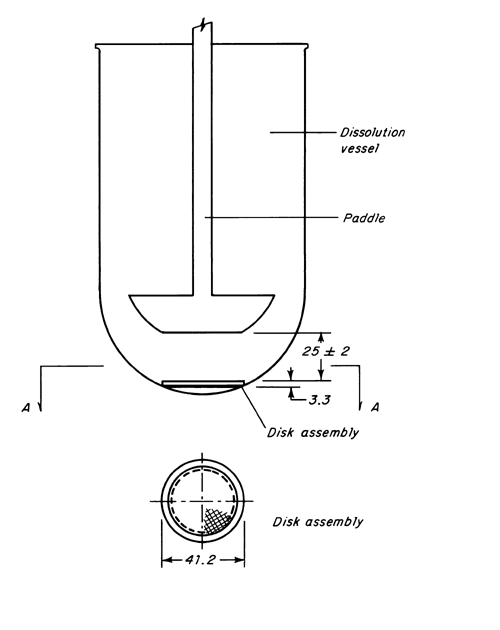
Apparatus 5 (Paddle over Disk)
Apparatus—
Use the paddle and vessel assembly from Apparatus 2 as described under Dissolution  711
711 , with the addition of a stainless steel disk assembly1 designed for holding the transdermal system at the bottom of the vessel. Other appropriate devices may be used, provided they do not sorb, react with, or interfere with the specimen being tested2. The temperature is maintained at 32 ± 0.5
, with the addition of a stainless steel disk assembly1 designed for holding the transdermal system at the bottom of the vessel. Other appropriate devices may be used, provided they do not sorb, react with, or interfere with the specimen being tested2. The temperature is maintained at 32 ± 0.5 . A distance of 25 ± 2 mm between the paddle blade and the surface of the disk assembly is maintained during the test. The vessel may be covered during the test to minimize evaporation. The disk assembly for holding the transdermal system is designed to minimize any “dead” volume between the disk assembly and the bottom of the vessel. The disk assembly holds the system flat and is positioned such that the release surface is parallel with the bottom of the paddle blade (see Figure 1).
. A distance of 25 ± 2 mm between the paddle blade and the surface of the disk assembly is maintained during the test. The vessel may be covered during the test to minimize evaporation. The disk assembly for holding the transdermal system is designed to minimize any “dead” volume between the disk assembly and the bottom of the vessel. The disk assembly holds the system flat and is positioned such that the release surface is parallel with the bottom of the paddle blade (see Figure 1).
Apparatus Suitability Test and Dissolution Medium—
Proceed as directed for Apparatus 2 under Dissolution  711
711 .
.
Procedure—
Place the stated volume of the Dissolution Medium in the vessel, assemble the apparatus without the disk assembly, and equilibrate the medium to 32 ± 0.5 . Apply the transdermal system to the disk assembly, assuring that the release surface of the system is as flat as possible. The system may be attached to the disk by applying a suitable adhesive3 to the disk assembly. Dry for 1 minute. Press the system, release surface side up, onto the adhesive-coated side of the disk assembly. If a membrane4 is used to support the system, it is applied so that no air bubbles occur between the membrane and the release surface. Place the disk assembly flat at the bottom of the vessel with the release surface facing up and parallel to the edge of the paddle blade and surface of the Dissolution Medium. The bottom edge of the paddle is 25 ± 2 mm from the surface of the disk assembly. Immediately operate the apparatus at the rate specified in the monograph. At each sampling time interval, withdraw a specimen from a zone midway between the surface of the Dissolution Medium and the top of the blade, not less than 1 cm from the vessel wall. Perform the analysis on each sampled aliquot as directed in the individual monograph, correcting for any volume losses, as necessary. Repeat the test with additional transdermal systems.
. Apply the transdermal system to the disk assembly, assuring that the release surface of the system is as flat as possible. The system may be attached to the disk by applying a suitable adhesive3 to the disk assembly. Dry for 1 minute. Press the system, release surface side up, onto the adhesive-coated side of the disk assembly. If a membrane4 is used to support the system, it is applied so that no air bubbles occur between the membrane and the release surface. Place the disk assembly flat at the bottom of the vessel with the release surface facing up and parallel to the edge of the paddle blade and surface of the Dissolution Medium. The bottom edge of the paddle is 25 ± 2 mm from the surface of the disk assembly. Immediately operate the apparatus at the rate specified in the monograph. At each sampling time interval, withdraw a specimen from a zone midway between the surface of the Dissolution Medium and the top of the blade, not less than 1 cm from the vessel wall. Perform the analysis on each sampled aliquot as directed in the individual monograph, correcting for any volume losses, as necessary. Repeat the test with additional transdermal systems.
Time—
The test time points, generally three, are expressed in hours. Specimens are to be withdrawn within a tolerance of ±15 minutes or ±2% of the stated time, the tolerance that results in the narrowest time interval being selected.
Interpretation—
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the system conform to Acceptance Table 1 for transdermal drug delivery systems. Continue testing through the three levels unless the results conform at either L1 or L2.
Acceptance Table 1
| Level | Number Tested | Criteria |
| L1 | 6 | No individual value lies outside the stated range. |
| L2 | 6 | The average value of the 12 units (L1 + L2) lies within the stated range. No individual value is outside the stated range by more than 10% of the average of the stated range. |
| L3 | 12 | The average value of the 24 units (L1 + L2 + L3) lies within the stated range. Not more than 2 of the 24 units are outside the stated range by more than 10% of the average of the stated range; and none of the units is outside the stated range by more than 20% of the average of the stated range. |
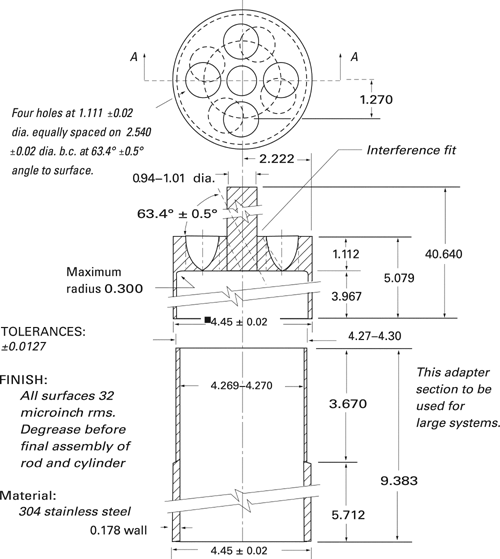
Apparatus 6 (Cylinder)
Apparatus—
Use the vessel assembly from Apparatus 1 as described under Dissolution  711
711 , except to replace the basket and shaft with a stainless steel cylinder stirring element and to maintain the temperature at 32 ± 0.5
, except to replace the basket and shaft with a stainless steel cylinder stirring element and to maintain the temperature at 32 ± 0.5 during the test. The shaft and cylinder components of the stirring element are fabricated of stainless steel to the specifications shown in Figure 2. The dosage unit is placed on the cylinder at the beginning of each test. The distance between the inside bottom of the vessel and the cylinder is maintained at 25 ± 2 mm during the test.
during the test. The shaft and cylinder components of the stirring element are fabricated of stainless steel to the specifications shown in Figure 2. The dosage unit is placed on the cylinder at the beginning of each test. The distance between the inside bottom of the vessel and the cylinder is maintained at 25 ± 2 mm during the test.
Fig. 2. Cylinder Stirring Element.5 (All measurements are expressed in cm unless noted otherwise.)
Dissolution Medium—
Use the medium specified in the individual monograph (see Dissolution  711
711 ).
).
Procedure—
Place the stated volume of the Dissolution Medium in the vessel of the apparatus specified in the individual monograph, assemble the apparatus, and equilibrate the Dissolution Medium to 32 ± 0.5 . Unless otherwise directed in the individual monograph, prepare the test system prior to test as follows. Remove the protective liner from the system, and place the adhesive side on a piece of Cuprophan4 that is not less than 1 cm larger on all sides than the system. Place the system, Cuprophan covered side down, on a clean surface, and apply a suitable adhesive 3 to the exposed Cuprophan borders. If necessary, apply additional adhesive to the back of the system. Dry for 1 minute. Carefully apply the adhesive-coated side of the system to the exterior of the cylinder such that the long axis of the system fits around the circumference of the cylinder. Press the Cuprophan covering to remove trapped air bubbles. Place the cylinder in the apparatus, and immediately rotate at the rate specified in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a quantity of Dissolution Medium for analysis from a zone midway between the surface of the Dissolution Medium and the top of the rotating cylinder, not less than 1 cm from the vessel wall. Perform the analysis as directed in the individual monograph, correcting for any volume losses as necessary. Repeat the test with additional transdermal drug delivery systems.
. Unless otherwise directed in the individual monograph, prepare the test system prior to test as follows. Remove the protective liner from the system, and place the adhesive side on a piece of Cuprophan4 that is not less than 1 cm larger on all sides than the system. Place the system, Cuprophan covered side down, on a clean surface, and apply a suitable adhesive 3 to the exposed Cuprophan borders. If necessary, apply additional adhesive to the back of the system. Dry for 1 minute. Carefully apply the adhesive-coated side of the system to the exterior of the cylinder such that the long axis of the system fits around the circumference of the cylinder. Press the Cuprophan covering to remove trapped air bubbles. Place the cylinder in the apparatus, and immediately rotate at the rate specified in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a quantity of Dissolution Medium for analysis from a zone midway between the surface of the Dissolution Medium and the top of the rotating cylinder, not less than 1 cm from the vessel wall. Perform the analysis as directed in the individual monograph, correcting for any volume losses as necessary. Repeat the test with additional transdermal drug delivery systems.
Time—
Proceed as directed under Apparatus 5.
Interpretation—
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the system conform to Acceptance Table 1 for transdermal drug delivery systems. Continue testing through the three levels unless the results conform at either L1 or L2.
Apparatus 7 (Reciprocating Holder)
note—This apparatus may also be specified for use with a variety of dosage forms.
Apparatus—
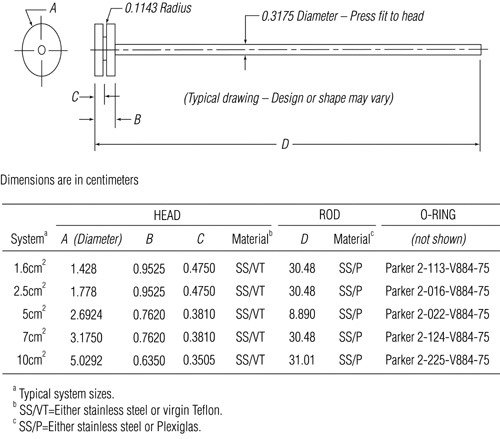
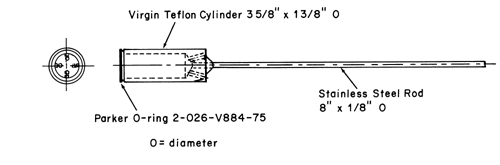
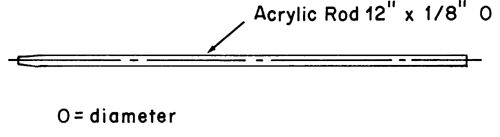
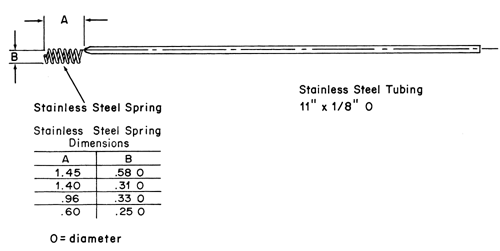
The assembly consists of a set of volumetrically calibrated or tared solution containers made of glass or other suitable inert material6, a motor and drive assembly to reciprocate the system vertically and to index the system horizontally to a different row of vessels automatically if desired, and a set of suitable sample holders (see Figure 37 and Figures 4a–4d). The solution containers are partially immersed in a suitable water bath of any convenient size that permits maintaining the temperature, T, inside the containers at 32 ± 0.5 or within the allowable range, as specified in the individual monograph, during the test. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating sample holder. Apparatus that permits observation of the system and holder during the test is preferable. Use the size container and sample holder as specified in the individual monograph.
or within the allowable range, as specified in the individual monograph, during the test. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating sample holder. Apparatus that permits observation of the system and holder during the test is preferable. Use the size container and sample holder as specified in the individual monograph.
Dissolution Medium—
Use the Dissolution Medium specified in the individual monograph (see Dissolution  711
711 ).
).
Sample Preparation A (Coated tablet drug delivery system)—
Attach each system to be tested to a suitable sample holder (e.g., by gluing system edge with 2-cyano acrylate glue onto the end of a plastic rod or by placing the system into a small nylon net bag at the end of a plastic rod or within a metal coil attached to a metal rod).
Sample Preparation B (Transdermal drug delivery system)—
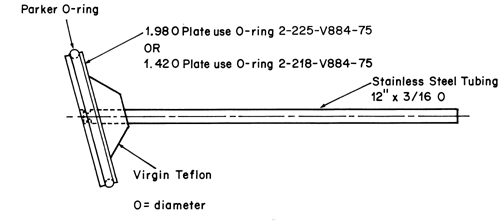
Press the system onto a dry, unused piece of Cuprophan4, nylon netting, or equivalent with the adhesive side against the selected substrate, taking care to eliminate air bubbles between the substrate and the release surface. Attach the system to a suitable sized sample holder with a suitable O-ring such that the back of the system is adjacent to and centered on the bottom of the disk-shaped sample holder or centered around the circumference of the cylindrical-shaped sample holder. Trim the excess substrate with a sharp blade.
Sample Preparation C (Other drug delivery systems)—
Attach each system to be tested to a suitable holder as described in the individual monograph.
Procedure—
Suspend each sample holder from a vertically reciprocating shaker such that each system is continuously immersed in an accurately measured volume of Dissolution Medium within a calibrated container pre-equilibrated to temperature, T. Reciprocate at a frequency of about 30 cycles per minute with an amplitude of about 2 cm, or as specified in the individual monograph, for the specified time in the medium specified for each time point. Remove the solution containers from the bath, cool to room temperature, and add sufficient solution (i.e., water in most cases) to correct for evaporative losses. Perform the analysis as directed in the individual monograph. Repeat the test with additional drug delivery systems as required in the individual monograph.
Interpretation—
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of the active ingredients released from the system conform to Acceptance Table 2 under Dissolution  711
711 for coated tablet drug delivery systems, to Acceptance Table 1 for transdermal drug delivery systems, or as specified in the individual monograph. Continue testing through the three levels unless the results conform at either L1 or L2.
for coated tablet drug delivery systems, to Acceptance Table 1 for transdermal drug delivery systems, or as specified in the individual monograph. Continue testing through the three levels unless the results conform at either L1 or L2.
1
Disk assembly (stainless support disk) may be obtained from Millipore Corp., Ashley Rd., Bedford, MA 01730.
2
A suitable device is the watchglass-patch-polytef mesh sandwich assembly available as the Transdermal SandwichTM from Hanson Research Corp., 9810 Variel Ave., Chatsworth, CA 91311.
3
Use Dow Corning, MD7-4502 Silicone Adhesive 65% in ethyl acetate, or the equivalent.
4
Use Cuprophan, Type 150 pm, 11 ± 0.5-µm thick, an inert, porous cellulosic material, which is available from Medicell International Ltd., 239 Liverpool Road, London NI ILX, England.
5
The cylinder stirring element is available from Accurate Tool, Inc., 25 Diaz St., Stamford, CT 06907, or from VanKel Technology Group, 13000 Weston Parkway, Cary, NC 27513.
6
The materials should not sorb, react with, or interfere with the specimen being tested.
7
The reciprocating disk sample holder may be purchased from ALZA Corp., 1900 Charleston Road, P.O. Box 7210, Mt. View, CA 94039–7210 or VanKel Technology Group.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | William E. Brown
Senior Scientist 1-301-816-8380 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 272
Pharmacopeial Forum: Volume No. 31(1) Page 213