Enoxaparin Sodium Injection
» Enoxaparin Sodium Injection is a sterile solution of Enoxaparin Sodium in Water for Injection. Its appearance is analyzed for clarity and degree of color using a validated method. Its potency value is not less than 90 percent and not more than 110 percent of the potency stated on the label in terms of International Anti-factor Xa Units (IU). It may contain, in multiple-dose containers, a suitable antimicrobial preservative, such as benzyl alcohol.
Packaging and storage—
Preserve in single-dose or multiple-dose containers in Type I glass. Store between 20 and 25
and 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Label it to indicate the amount (mg) of Enoxaparin Sodium in the total volume of contents. The label states also that the Enoxaparin Sodium starting material is porcine derived.
USP Reference standards  11
11 —
—
USP Benzyl Alcohol RS .
.
USP Endotoxin RS.
USP Enoxaparin Sodium RS .
.
USP Enoxaparin Sodium Solution for Bioassays RS.
USP Benzyl Alcohol RS
 .
.
USP Endotoxin RS.
USP Enoxaparin Sodium RS
 .
.
USP Enoxaparin Sodium Solution for Bioassays RS.
Identification—
A:
Add 2 mL of water to the total content of a single-dose container, or to 0.4 mL from a multiple-dose container, and 1 mL of 2% w/v protamine sulfate solution in a glass test tube, and mix. A creamy white precipitate is formed.
B: Ultraviolet Absorption  197U
197U —
—
Standard solution:
500 µg per mL.
Medium:
0.01 N hydrochloric acid. The spectra exhibit maxima at 231 ± 2 nm.
Test solution—
Transfer the total content of a single-dose container, or 0.4 mL from a multiple-dose container, to a 100-mL volumetric flask. Dilute with Medium to volume.
C:
It meets the requirements of the test for Sodium  191
191 .
.
pH  791
791 :
between 5.5 and 7.5.
:
between 5.5 and 7.5.
Benzyl alcohol content (if present)—
Mobile phase—
Prepare a filtered and degassed mixture of water, acetonitrile, and methanol (80:15:5 v/v).
Standard solution—
Transfer about 75 mg, accurately weighed, of USP Benzyl Alcohol RS to a 50-mL volumetric flask, and dilute with Mobile phase to volume.
Test solutions—
Transfer 5.0 mL of the Injection to a 50-mL volumetric flask. Dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 256-nm detector and a 4.6-mm × 15-cm stainless steel column that contains packing L7. 1 The flow rate is about 1.0 mL per minute maintained constant to ±10%.
)—
The liquid chromatograph is equipped with a 256-nm detector and a 4.6-mm × 15-cm stainless steel column that contains packing L7. 1 The flow rate is about 1.0 mL per minute maintained constant to ±10%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution, record the chromatograms, and measure the peak responses. Calculate the percentage of benzyl alcohol in the portion of enoxaparin sodium solution taken by the formula:
(AT × M)/(AS × 200)
in which AT and AS are areas of the benzyl alcohol peaks in the chromatograms of the Test solution and the Standard solution, respectively; and M is the mass of the benzyl alcohol dissolved to prepare the Standard solution. The percentage (w/v) of benzyl alcohol in the Injection is not less than 1.35% and not more than 1.65%.
Bacterial endotoxins  85
85 —
It contains less than 0.01 USP Endotoxin Unit per unit of anti-factor Xa activity in Anti-factor Xa IU.
—
It contains less than 0.01 USP Endotoxin Unit per unit of anti-factor Xa activity in Anti-factor Xa IU.
Free sulfate content—
Mobile phase—
Prepare a 3.0 mM sodium carbonate solution. Make adjustments if necessary.
Standard sulfate stock solution—
Prepare a solution of sodium sulfate in Mobile phase in a suitable sulfate-free container such that the concentration of sulfate is accurately known at about 1 g per L. Transfer about 5 g, accurately weighed, of the solution to a similar container, and add Mobile phase to obtain about 25 g of solution.
Standard solutions—
Prepare standard solutions at concentrations of 0.1 µg per g, 0.5 µg per g, 1 µg per g, 2 µg per g, 4 µg per g, and 5 µg per g by appropriate dilution of the Standard sulfate stock solution in Mobile phase.
System suitability solution—
Prepare a solution containing 3 µg per mL of sulfate anion and 5 µg per mL of oxalate anion.
Test solutions—
Transfer about 200 mg of a 100 mg per mL Injection, accurately weighed, to a suitable previously tared sulfate-free vial. Add Mobile phase to obtain a total mass, MS, of about 20 g.
Chromatographic system (see Chromatography  621
621 )—
The ion chromatograph is equipped with a conductivity detector and a 4-mm × 5-cm anion-exchange guard column, a 4-mm × 25-cm anion-exchange analytical column, both containing L61 packing (see Chromatographic Reagents under Reagents, Indicators, and Solutions), and a micromembrane anion autosuppressor2 or a suitable chemical suppression system. The flow rate is about 2.0 mL per minute.
)—
The ion chromatograph is equipped with a conductivity detector and a 4-mm × 5-cm anion-exchange guard column, a 4-mm × 25-cm anion-exchange analytical column, both containing L61 packing (see Chromatographic Reagents under Reagents, Indicators, and Solutions), and a micromembrane anion autosuppressor2 or a suitable chemical suppression system. The flow rate is about 2.0 mL per minute.
Procedure—
Chromatograph about 25 µL of the System suitability solution. The resolution between the sulfate and oxalate peaks is greater than 1. Separately inject 25 µL of the Standard solutions and the Test solution into the chromatograph, and plot the standard curve of sulfate peak height as a function of sulfate concentration (in µg per g) in the Standard solutions. From the sulfate peak height in the chromatogram determine the concentration of sulfate, T, in µg per g, in the Test solution using the standard curve. Calculate the percentage of free sulfate content (w/w) in the Injection taken by the formula:
T × MS / 10m
in which m is the mass, in mg, of Injection aliquoted to prepare the Test solution. The percentage of free sulfate is not more than 0.12%.
Assay (anti-factor Xa activity)—
Proceed as directed for Assay (anti-factor Xa activity) under Enoxaparin Sodium.
Anti-factor Xa to anti-factor IIa ratio—
The ratio of the numerical value of the anti-factor Xa activity in Anti-Factor Xa IU per mg to the numerical value of the anti-factor IIa activity in Anti-Factor IIa IU per mg, as determined by the Assay (anti-factor Xa activity) and the Anti-factor IIa activity, respectively, is not less than 3.3 and not more than 5.3.
Other requirements—
It meets the requirements under Injections  1
1 , Particulate Matter in Injections
, Particulate Matter in Injections  788
788 , and Sterility Tests
, and Sterility Tests  71
71 .
.
1
Available as Lichrospher 100 RP 18, Pore size 100 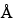 , Particle size 5 µm, or equivalent.
, Particle size 5 µm, or equivalent.
2
Available as Anion Self-Regenerating Suppressor (ASRS) from Dionex Inc, or equivalent.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Anita Y. Szajek, Ph.D.
Senior Scientist 1-301-816-8325 |
(BBBBP05) Biologics and Biotechnology - Blood and Blood Products |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2256
Pharmacopeial Forum: Volume No. 33(1) Page 58
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.